Every organism has to cope with an assortment of predators and parasites.
Many biologists feel that one of the most potent forces of evolutionary adaptation is that created by the interactions between a host and its parasites.
As the host evolves measures that give it greater protection from a parasite, the parasite evolves countermeasures.
This page examines some of the mechanisms by which parasites evade host defense measures. The emphasis will be on human parasites.
Most parasites enter their human host through
- food and water
- air
- the bites of arthropod vectors like mosquitoes, fleas, and ticks.
However, for categories 1 and 2, entry begins in only a superficial sense. The gastrointestinal tract, lungs, and genitourinary tract are simply part of the outside folded in. Parasites that arrive there are still outside the internal environment of the body.

Some organisms are content with this. Some of our epithelial surfaces (e.g., nasopharynx, colon) are colonized by a vast array of microorganisms. Most are commensals, usually living harmlessly in these sites. Some, however, may gain the upper hand and cause disease in individuals with weakened immune systems (e.g., in AIDS patients).
A few are pathogenic.
Two examples:
- Neisseria gonorrhoeae, the bacterium that causes gonorrhea. In the U.S., this is one of the most common sexually-transmitted diseases (STDs). The bacteria live on the surface of the mucous membranes of the genitourinary tract.
Their presence does induce antibodies (of the IgA class), but these fail to cure the host.
The reason: the bacteria periodically change a protein, called pilin, on their surface so that epitopes that the immune system has learned to produce antibodies against disappear and are replaced by epitopes that the immune system must respond to anew. The epitopes are encoded by a family of diverse gene fragments. Periodically one of these fragments gets inserted into the remainder of the pilin gene. This hypervariable region encodes a new amino acid sequence at the C-terminal of the pilin molecule (analogous to the hypervariable regions of antibodies) which will not be recognized by the current crop of antibodies (but will, in turn, eventually elicit a new set of antibodies).
- Helicobacter pylori. This in the only bacterium known that can live in the harsh environment (pH ~ 1.4) of the stomach. It survives by taking up urea and converting it into ammonia to neutralize the acid. H. pylori is the main cause of stomach ulcers. To do so, it must be able not only to survive in the stomach but to invade the internal environment.
To get into the true interior of the body, invading parasites must pass through an epithelial barrier. For those injected by biting vectors, this is no problem.
But the others must find a trick. One of these: Helicobacter pylori secretes a protein that disrupts the tight junctions and adherens junctions that seal epithelial cells.
Once within the body, intracellular pathogens, e.g., viruses and some bacteria and protists, must have a mechanism to invade their host cells.
Some tricks:
- Streptococcus pneumoniae. Epithelial cells like those in the nasopharynx have receptors that are responsible for transporting IgA and IgM antibodies from the blood to the apical surface of the cell. The pneumococcus piggybacks on this receptor on its return trip into the cell. (This is the organism that led to the discovery that genes are DNA. Link to a discussion.)
- Vaccinia virus. Vaccinia virus, the agent used to vaccinate against smallpox, leaves its host cell by budding — enveloping itself in host plasma membrane (like the budding in this view of a different virus). Because infection has caused the cell to begin to die by apoptosis, the phospholipid phosphatidylserine — normally expressed exclusively on the inner layer of the lipid bilayer — is now displayed on the surface of the budding virions. Phosphatidylserine is a powerful "eat me" signal, which stimulates other cells to engulf what looks like a piece of cell debris but is an infectious virus particle instead. By co-opting a normal cellular process, the virus is able to quickly spread from cell to cell. Perhaps other enveloped viruses, like HIV, use a similar trick.
- Listeria monocytogenes. This food-borne bacterium [link to its genome] can be dangerous to people with defective immune systems as well as to pregnant women and their newborn babies. It has two kinds of surface molecules, each a ligand for a different receptor on the cell surface, and uses these to enter the cell by receptor-mediated endocytosis.
- Salmonella enterica var. Typhi. This bacterium, the cause of typhoid fever, also enters cells by receptor-mediated endocytosis. It binds to the CFTR molecules on the surface of the host cell. Perhaps the relatively high frequency of some mutant CFTR alleles (e.g. the deletion of Phe-508 — see Patient E) that cause cystic fibrosis when homozygous) is that they protect the heterozygous host from invasion by typhoid bacilli. [Link to discussion of balanced polymorphism.]
- Pathogenic mycobacteria. These agents (e.g., Mycobacterium tuberculosis, the agent of tuberculosis) bind the complement fragment C2a forming a C3 convertase that opsonizes the bacteria for phagocytosis by the macrophages that patrol the lung surfaces. [Link to discussion of the complement system]
Using phagocytic cells, like macrophages and neutrophils, to gain entry into the internal environment is a risky strategy because phagocytes are poised to destroy engulfed bacteria. However, some bacteria have evolved mechanisms to avoid destruction even after they have been engulfed by phagocytes.
Three examples:
- Salmonella enterica. Once engulfed by phagocytosis, it secretes a protein that prevents the phagosome containing it from fusing with a lysosome.
- Mycobacteria (e.g., the tubercle bacillus that causes tuberculosis) use a different trick.
- When the phagosome is first pinched off from the plasma membrane, it is coated with a protein called "TACO" (for tryptophan-aspartate-containing coat protein).
- This must be removed before the phagosome can fuse with a lysosome.
- Mycobacteria taken into a phagosome are able, in some way, to keep the TACO coat from being removed.
- Thus there is no fusion with lysosomes, and the mycobacteria can continue to live in this protected intracellular location (unless destroyed by the backup system of autophagy).
- Listeria monocytogenes. Once engulfed by a macrophage, the bacteria secrete a protein that destroy the phagosome membrane thus releasing the bacteria into the cytoplasm of the cell where they proliferate happily (unless destroyed by the backup system of autophagy).
As their numbers increase, they polymerize actin monomers of their host forming actin filaments (aka "microfilaments"). They use these to move to the periphery of the cell where they enter protrusions called filopods. These are taken up by adjacent cells thus allowing the organisms to spread through the tissue safe from exposure to antibodies.
Parasites that live in the blood or the interstitial fluid that bathes cells are at risk of being destroyed by antibodies directed against them.
A common countermeasure is to periodically alter the epitopes on the parasite surface so that antibodies can no longer recognize them.
Examples:
 Trypanosomes are flagellated protozoans that swim in the blood. (This photomicrograph is courtesy of Turtox.) In humans they cause trypanosomiasis or African sleeping sickness.
Trypanosomes are flagellated protozoans that swim in the blood. (This photomicrograph is courtesy of Turtox.) In humans they cause trypanosomiasis or African sleeping sickness.
Once introduced into the blood (from the bite of an infected tsetse fly), they multiply rapidly and may soon reach a population density of >106 organisms per milliliter.
Soon, however, this number drops rapidly (remission) only to be followed a week or two later by another wave of population growth (relapse). This pattern continues indefinitely.
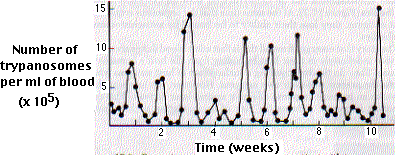 |
| Recurring waves of parasitemia in a patient with sleeping sickness. Each peak represents the development of a clone of trypanosomes expressing a new variant surface glycoprotein (VSG). [From K. Vickerman, Ciba Foundation Symp. 25:53, 1974.] |
The periods of remission are caused by the appearance of antibodies directed against the glycoprotein molecules that coat the organisms.
The relapses are caused by the appearance of a small number of parasites that express a new version of the glycoprotein coat (designated VSG for variant surface glycoprotein).
- Trypanosomes contain a repertoire of over 2,000 VSG genes, but only one of these is expressed at a time.
- The gene to be expressed is copied, and the copy is moved to an active site on the chromosome near a telomere. (The mechanism resembles the type of copy-paste transposition found in some transposons.)
- Here the duplicated copy is transcribed and translated into the VSG protein.
The switch to a new active VSG gene is probably a chance event. However, if its owner is bathed in antibodies against the earlier dominant VSG, it now has a competitive advantage that allows it to propagate until it, too, has to face an immune response from the host.
 This autoradiogram (Courtesy of P. Borst; from P.A.M. Michels, et al., Nucleic Acids Research 10:2353, 1982.) shows Southern blots of nuclear DNA from clones of Trypanosoma brucei expressing different variant surface glycoproteins (VSGs). The DNA was digested with the same restriction enzyme (PstI) in every case. The probe was a fragment of cDNA from a clone designated 118.
This autoradiogram (Courtesy of P. Borst; from P.A.M. Michels, et al., Nucleic Acids Research 10:2353, 1982.) shows Southern blots of nuclear DNA from clones of Trypanosoma brucei expressing different variant surface glycoproteins (VSGs). The DNA was digested with the same restriction enzyme (PstI) in every case. The probe was a fragment of cDNA from a clone designated 118.
- The probe binds to a 2.1 kb fragment in every clone examined showing that all have one copy of the 118 gene.
- It also binds to a second fragment in the clone shown in lanes 3 and 8 (they are duplicates) which express the VSG-118 protein on their surface using their duplicated copy of the 118 gene.
- The clone in lane 9, which also expresses the 118 gene, is a descendant of the clone in lane 6 which did not (single-headed arrow).
- The clones in lanes 6 and 7 were derived from the 118-expressing clone in lane 3, but no longer have the second copy of the gene (double-headed arrow) and so no longer express the 118 VSG.
This sporozoan is the most dangerous of the several that cause malaria. Like the others, it spends most of its life in the human within red blood cells (RBCs).
One might think that tucked within an RBC, it would be safe from antibodies in the plasma. But, in fact, the parasite synthesizes a protein that appears at the surface of the RBC. These molecules anchor the RBCs to the walls of the blood vessels so they won't be swept into and destroyed by the host spleen.
The surface protein also elicits an immune response. To evade this problem, the parasite periodically alters the composition of the protein so that it is no longer recognized by the current crop of antibodies. It does this by switching to expressing another one of the 60 genes that encode variants of this protein.
| Plasmodium falciparum plays another game. During the period when its gametocytes — the stage that infects the mosquito vector — are present, infected children exude some airborne attractant that lures Anopheles mosquitoes to them. |
Although an intracellular parasite, new HIV virions are exposed to blood and lymph as they are escape from dying cells and must find a new cell to infect.
Although the immune system mounts an antibody response to them (the basis of the most common test for HIV infection), this response does not lead to a cure.
- One reason: the reverse transcriptase of HIV is prone to making coding errors. These can change the envelope protein, producing a new epitope that the immune system hasn't seen before. So as the course of infection progresses in an AIDS patient, an increasingly diverse population of HIV clones develops — another reason why it has been so difficult to come up with an effective vaccine.
- A second reason: the surface proteins on the virions (gp120 and gp41) are glycoproteins whose sugar groups have been placed on the proteins by host cell enzymes and thus resemble "self".
As their name suggests, these flatworms live in the blood, taking up final residence in the veins draining the
- large intestine (Schistosoma mansoni),
- small intestine (Schistosoma japonicum), or
- urinary bladder (Schistosoma haematobium).
Their presence induces antibodies that — with the aid of macrophages and eosinophils —
- protects the host from infection by a fresh crop of invaders but
- are incapable of harming the already-established worms.
It appears that the resident worms coat themselves with host antigens
and thus disguise themselves as normal components of the body ("self").
 The bacterium Neisseria meningitidis is one of the major causes of bacterial meningitis, a life-threatening infection of the meninges.
This organism has at least two tricks by which it evades antibody-mediated immunity.
The bacterium Neisseria meningitidis is one of the major causes of bacterial meningitis, a life-threatening infection of the meninges.
This organism has at least two tricks by which it evades antibody-mediated immunity.
- Neisseria meningitidis (as well as its relative Neisseria gonorrhoeae) secrete a protease that cuts human IgA1 molecules into their Fab (recognition) fragments and their Fc (effector) fragment. Thus separated, these antibodies — which coat the mucosal surfaces that Neisseria invades — can no longer do their job. Curiously, we secrete a second type of IgA (IgA2) which lacks a hinge region and thus is not destroyed by Neisseria's protease (a counter-countermeasure?)
- Neisseria meningitidis also eludes antibody-mediated immunity by producing a protein that binds Factor H of the complement system. Factor H normally binds to the sugar molecules on human cells protecting them against attack by the complement system — the system that destroys antigens that have been bound by antibodies [Link]. By coating itself with Factor H, Neisseria meningitidis gains similar protection.
Many viruses (all of which are intracellular parasites) exploit receptor-mediated endocytosis to sneak their way into their host cell.
They have evolved surface molecules that serve as decoy ligands for receptors on the target cell surface. Binding to these receptors tricks the cell into engulfing the virus.
Some examples:
- Epstein-Barr Virus (EBV). This virus causes mononucleosis and is a contributing factor in the development of Burkitt's lymphoma, a cancer of B lymphocytes. It binds to receptors present on the surface of B cells.
- HIV, the human immunodeficiency virus. It binds to the CD4 molecule on the surface of the CD4+ subset of T cells.
- Influenza virus. The hemagglutinin on the surface of the virus binds to carbohydrate on the target cell surface tricking the cell into engulfing it [More].
The process can be remarkably fast. A team in Munich (Seisenberger et al., Science, 30 November 2001) succeeded in attaching single fluorescent molecules to single virions of adeno-associated virus (AAV) and watched them infect a HeLa cell:
- After bumping against the host cell's plasma membrane an average of 5 times,
- the virus was engulfed in an average of 64 milliseconds;
- took 15 minutes to pass (in the endosome) through the cytosol and
- enter the nucleus.
Once within a cell, a virus is safe from attack by antibodies. But it is still subject to being destroyed by an attack by CD8+ cytotoxic T lymphocytes (CTL). Fragments of proteins synthesized by the virus will be deposited in the groove of class I histocompatibility molecules and displayed at the cell surface. These will be recognized as "foreign" and elicit an attack which will destroy the host cell along with its content of viruses.
Some countermeasures:
- Herpes Simplex Virus-1 (HSV-1).
This agent (which causes "cold sores") avoids detection by
- turning off all its protein-coding genes so no potential antigen is displayed at the host cell surface;
- turning on a single gene whose RNA transcript is converted into a microRNA (miRNA) by the host cell. This miRNA inhibits apoptosis of the host cell so that the virus remains safely inside forming a "latent" infection.
- Human Cytomegalovirus (HCMV). It synthesizes 2 proteins that interrupt the class I pathway by sending MHC class I molecules to be degraded in proteasomes before they can deliver viral antigen fragments to the cell surface. It also encodes 2 microRNAs — one that protects the host cell from being killed by natural killer cells and one that protects the cell from apoptosis.
- Epstein-Barr Virus (EBV) also becomes dormant (in B cells) reducing the number of proteins it synthesizes from ~100 to only 8. Furthermore, one of these is a molecule that mimics the lymphokine IL-10 thus inhibiting the Th1 cells that support cell-mediated immunity.
- Adenoviruses. These agents (of the many responsible for the common cold) protect themselves from cell-mediated immune attack by inhibiting the display of class I histocompatibility molecules on surface of the cell in which they reside. HIV-1, the cause of AIDS, uses the same trick.
One basic rule is that a parasite must not kill its host before the parasite has succeeded in reproducing and moving on to a new host.
Many parasites leave their host by the same route by which they entered:
- air (e.g., influenza)
- insect bites (e.g., malaria, sleeping sickness)
- out the other end of the gastrointestinal tract in the feces (e.g., typhoid bacillus)
For some, completing their life cycle requires that they sequentially parasitize one or more alternate hosts. While this may seem to complicate their lives, in every case it actually increases their chances for survival. (Humans are more likely to eat an infected fish than their own feces.)
| Some parasite life cycles involving multiple hosts:
|
Some parasites alter the behavior of their host in ways that increase their chances of moving on to a new host. These are parasites that need an alternate host in order to complete their life cycle.
Some examples:
- a fluke (Phylum Platyhelminthes) that causes its periwinkle (mollusk) host to move up close to the surface in the intertidal zone where it is more likely to be eaten by the sea gull that serves as the alternate host;
- another fluke that causes its fish host to jump about making it more noticeable to the birds that prey on it;
- still another fluke (Dicrocoelium dendriticum) that causes its ant intermediate host to attach itself high on a blade of grass where it is more likely to be eaten by its final host (cow, sheep, etc.).
- Toxoplasma gondii, a cat intestinal parasite. The normal intermediate host for T. gondii is the rat. Infected rats lose their natural fear of cats increasing the chance that the parasite will move on to another cat.
T. gondii also parasitizes humans (e.g, acquired from contact with kitty litter). This is seldom a problem although it can be dangerous to the fetus of pregnant women and to people with weakened immune systems (e.g., AIDS patients). Some psychologists claim that infected humans show personality changes as well.
The relative success of the measures and countermeasures taken by parasites and their host affects the dynamics of their association. For humans, it results in illnesses ranging from acute to chronic.
Acute Infections
In these, the parasite must move on to a new host before it either kills — or is killed by — its present host. Some examples of these "hit-and-run" diseases: influenza, smallpox, polio, measles.
Characteristics:
- Cause an acute illness of short duration.
- Contagious for only a brief period.
- Disease ends in the elimination of the parasite (with or without the death of its host).
- If the host survives, it usually has acquired a lifelong immunity that will prevent reinfection by that parasite.
- Continued survival of the parasite depends on it quickly finding other susceptible hosts.
- These diseases flourish best in cities and other crowded places (e.g., military camps, college dormitories).
- These diseases are responsible for most of the great epidemics in human history
- the black death (caused by Yersinia pestis) of the Middle Ages.
- the plagues (measles?) that decimated the Native American population after the arrival of the first European explorers.
- the measles epidemics that decimated the population of Polynesia in the nineteenth century.
- the great influenza pandemic of 1918–1919. [More]
- Vaccination programs can eliminate epidemics of the disease even if 100% of the population is not reached. This "herd immunity" results from a reduction in the number of susceptible targets below the level needed to sustain the epidemic. A worldwide vaccination program eliminated smallpox from the earth in the 1970s. A similar program against polio is getting close to that goal.
Chronic Infections
In these, the parasite survives for long periods without either killing or being killed by its host. Some examples: malaria, trypanosomiasis, tuberculosis, leprosy, schistosomiasis.
Characteristics:
- The parasite remains in the host for a long time (often for the host's entire life).
- The host remains contagious throughout this time.
- The parasite evades the immune responses of the host.
- The parasite can persist in small, isolated populations.
- Vaccination programs are unlikely to produce herd immunity.
Microorganisms that live harmlessly within our body (commensalism), or even benefit us (mutualism) are nonetheless seen as foreign by the immune system and are at risk of attack by it. How they avoid attack is a mystery for most, but is understood for one common inhabitant of the human colon, Bacteroides fragilis.
This bacterium coats itself in a polysaccharide capsule (much the way that the pneumococcus does — Link). Unlike the pneumococcus, however, B. fragilis periodically switches the chemical composition of its capsule by switching on a different gene needed for its synthesis. By periodically changing the surface exposed to antibodies, the bacterium avoids being damaged by them (the same strategy used by trypanosomes).
5 November 2018


 Trypanosomes are flagellated protozoans that swim in the blood. (This photomicrograph is courtesy of Turtox.) In humans they cause trypanosomiasis or African sleeping sickness.
Trypanosomes are flagellated protozoans that swim in the blood. (This photomicrograph is courtesy of Turtox.) In humans they cause trypanosomiasis or African sleeping sickness.

 This autoradiogram (Courtesy of P. Borst; from P.A.M. Michels, et al., Nucleic Acids Research 10:2353, 1982.) shows Southern blots of nuclear DNA from clones of Trypanosoma brucei expressing different variant surface glycoproteins (VSGs). The DNA was digested with the same restriction enzyme (PstI) in every case. The probe was a fragment of cDNA from a clone designated 118.
This autoradiogram (Courtesy of P. Borst; from P.A.M. Michels, et al., Nucleic Acids Research 10:2353, 1982.) shows Southern blots of nuclear DNA from clones of Trypanosoma brucei expressing different variant surface glycoproteins (VSGs). The DNA was digested with the same restriction enzyme (PstI) in every case. The probe was a fragment of cDNA from a clone designated 118.
 The bacterium Neisseria meningitidis is one of the major causes of bacterial meningitis, a life-threatening infection of the meninges.
This organism has at least two tricks by which it evades antibody-mediated immunity.
The bacterium Neisseria meningitidis is one of the major causes of bacterial meningitis, a life-threatening infection of the meninges.
This organism has at least two tricks by which it evades antibody-mediated immunity.