Sometimes the interaction of antibodies with antigen is useful by itself.
For example,
- coating a virus or bacterium thus preventing it from binding to — and invading — a host cell (e.g., antipolio antibodies);
- binding to a toxin molecule (e.g., diphtheria or tetanus toxin) thus keeping the toxin from entering a cell where it does its dirty work.
But most of the time, the binding of antibodies to antigen performs no useful function until and unless it can activate an effector mechanism. The complement system serves several effector roles.
So,
- the complement system provides the actual protection from the response while
- the interaction of antibodies and antigen provides the specificity of the response.
- Put another way, antibodies "finger" the target, complement destroys it.
Features of the system
- The complement system consists of some 30 proteins circulating in blood plasma.
- Most of these are inactive until
- they are cleaved by a protease which, in turn,
- converts them into a protease.
- Thus many components of the system serve as the substrate of a prior component and then as an enzyme to activate a subsequent component.
- This pattern of sequential activation produces an expanding cascade of activity (reminiscent of the operation of the blood clotting system [Link]).
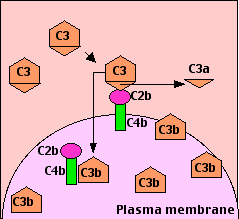
The binding of antibody to its antigen often triggers the complement system through the so-called classical pathway. It can occur in solution or — as shown here — when the antibodies have bound to antigens on a cell surface.
The proteins of the classical pathway
C1 exists in blood serum as a molecular complex containing:

- 6 molecules of C1q
- 2 molecules of C1r
- 2 molecules of C1s
The constant regions of mu chains (IgM) and some gamma chains (IgG) contain a binding site for C1q. A single molecule of IgM is enough to initiate the pathway. IgG is far less efficient, requiring several molecules to do so (6 is the optimum — the same as the number of C1q molecules in C1).
- Binding of C1q activates C1s and C1r.
- Activated C1s (a serine protease) cleaves two serum proteins:
- C4 is cleaved into a large fragment
- C4b, which binds covalently to sugar residues on cell-surface glycoproteins, and a smaller, inactive, fragment of
- C4a which diffuses away.
- C2 is cleaved into
- C2b, which binds noncovalently to a site on C4b, leaving a smaller, inactive, fragment of
- C2a which diffuses away.
- The complex of C4b•2b is called "C3 convertase" because it catalyzes the cleavage of C3. (C4b•2b is also a serine protease.)
| (Over a decade ago, complement workers decided that the smaller of all C fragments should be designated with an "a", the larger with a "b". This required changing the existing nomenclature for C2. That is why you will find the C3 convertase designated C4b•2a in older literature.) |
 C3 is the most abundant protein of the complement system (~1.3 mg/ml).
Because of its abundance and its ability to activate itself (by a mechanism described below), it greatly magnifies the response.
C3 is the most abundant protein of the complement system (~1.3 mg/ml).
Because of its abundance and its ability to activate itself (by a mechanism described below), it greatly magnifies the response.
- C4b•2b cuts C3 into two major fragments:
- C3b, which binds covalently to glycoproteins scattered across the cell surface. Macrophages and neutrophils have receptors for C3b and can bind the C3b-coated cell or particle preparatory to phagocytosis. This effect qualifies C3b as an opsonin.
| As Dr. Ridgeon says in George Bernard Shaw's play The Doctor's Dilemma, "Opsonin is what you butter the disease germs with to make your white blood corpuscles eat them." |
- C3a This small fragment is released into the surrounding fluids. It can bind to receptors on basophils and mast cells triggering them to release their vasoactive contents (e.g., histamine). Because of the role of these materials in anaphylaxis, C3a is called an anaphylatoxin.
- Some of the C3b binds to molecules of C5 creating an allosteric change that exposes them to cleavage by C4b•2b (which is thus a "C3/C5 convertase".)
Cleavage of C5 by the C3/C5 convertase initiates the assembly of a set of complement proteins that make up the membrane attack complex. (The membrane attack complex can also be formed by another C5 convertase produce by the "alternative pathway" [Link].)
Cleavage of C5 by the C3/C5 convertase, produces:
- C5a, which is released into the fluid surroundings where it
- C5b, which serves as the anchor for the assembly of a single molecule each of
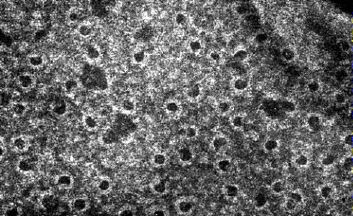
- The resulting complex C5b•6•7•8 guides the polymerization of as many as 18 molecules of C9 into a tube inserted into the lipid bilayer of the plasma membrane. This tube forms a channel allowing the passage of ions and small molecules. Water enters the cell by osmosis and the cell lyses.
The electron micrograph (courtesy of Drs. J. H. Humphrey and R. Dourmashkin) shows holes punched through the cell wall of the Gram-negative bacterium Shigella dysenteriae by the terminal components of the complement system. (Some of the holes are larger than expected for C9 channels and probably were enlarged later by the action of lysozyme.)
Cell lysis is only one function (and probably not the most important one) of the complement system.
The complement system acts in several ways to mobilize defense mechanisms.
- Opsonization by C3b targets foreign particles for phagocytosis.
- Chemotaxis by C5a attracts phagocytic cells to the site of damage.
- This is aided by the increased permeability of the capillary beds mediated by C3a and C5a.
- The early complement components are also important for solubilizing antigen-antibody complexes assisting in their catabolism and elimination from the body. Failure of this function can lead to immune complex disorders.
- Lysis of antibody-coated cells. (In some cases, this causes more harm than good; complement-mediated lysis can cause such serious disorders as
- Promoting antibody formation. Breakdown of C3b generates a fragment (C3d) that binds to antigens enhancing their uptake by dendritic cells and B cells. [Link to a discussion of antigen presentation.]
(The C3d.antigen complex binds to the same receptor on B cells that the Epstein-Barr virus (EBV) uses to gain entry into B cells — where it may cause mononucleosis and, sometimes, Burkitt's lymphoma.)
The complement system can also be triggered without antigen-antibody complexes.
Even in their absence, there is a spontaneous conversion of C3 to C3b. Ordinarily the C3b is quickly inactivated: the C3b binds to inhibitory proteins and sialic acid present on the surface of the body's own cells, and the process is aborted.
However, bacteria and other foreign materials that may get into the body lack these proteins and have little or no sialic acid. So the C3b
- binds a protein called Factor B forming a complex of C3b•Bb.
- C3b•Bb is also a C3 convertase acting on more C3 to form:
- C3b•Bb•C3b, which is a C5 convertase and can start the assembly of the membrane attack complex.
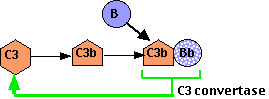
- more C3b! This second function (shown here) creates a positive feedback loop, amplifying what might have started as a small reaction (the formation of C3b by either or both the classical and alternative pathways) into a massive production of C3b.
The explosive potential of the complement system requires that it be kept under tight control.
At least 12 proteins are known that do this. Three examples:
- Factor H removes Bb from the alternative pathway C3 convertase breaking the positive feedback loop.
- Factor I inactivates C3b.
- C1 inhibitor (C1INH) binds to sites on activated C1r and C1s shutting down their proteolytic activity. So when C1 is activated by antigen-antibody complexes, there is only a brief interval during which it can cleave C4 and C2 before it is deactivated by C1INH.
With so many proteins involved, it is not surprising that inherited deficiencies of one or another are sometimes encountered in humans.
Five examples:
- C3. An inherited deficiency of C3 predisposes the person to frequent bouts of bacterial infections.
- C2. Curiously, immune complex disorders, not bacterial infections, are the main problem with a deficiency of C2 (or of one of the other "early" components like C1q, C1r, C1s, or C4). This emphasizes the important role of the complement system in clearing away antigen-antibody complexes.
- C1q. A deficiency of C1q is frequently found in patients with the autoimmune disorder systemic lupus erythematosus (SLE).
- C9. Another curiosity: most people who cannot make C9 have no more of a problem with bacterial infections than those who can. Laboratory studies suggest that the C5b•6•7•8 complex by itself is able to lyze bacteria although not as efficiently as C9. And, in fact, a deficiency of C8 is associated with a sharply-increased risk of bacterial meningitis.
- C1INH. A deficiency of C1INH produces hereditary angioedema (HAE). [Link to mechanism]
17 March 2020




