Reaching the goal of effective gene therapies for human diseases has been a difficult one.
Some of the problems that remain to be solved include:
- how to avoid an immune response in the patient, which can interfere with gene therapy in two ways:
Both of these are serious problems with adenovirus vectors.
- how to get the gene into non-dividing cells like liver, muscle, and neurons;
- how to get the gene to be replicated (in dividing cells) and expressed indefinitely but
- minimize the risk that it inserts near a proto-oncogene which it could activate producing a cancer. (This occurred in several little boys treated with a retroviral vector based on the murine leukemia virus. [Link])
- how to get the gene to be expressed as needed; that is, how to bring the gene under normal physiological controls so that its product is produced where, when, and in the amounts needed.
Adeno-associated virus gets its name because it is often found in cells that are simultaneously infected with adenovirus. However, by itself it seems to be harmless.
Unlike adenovirus, AAV
- does not stimulate inflammation in the host;
- can enter non-dividing cells;
- integrates successfully into one spot in the genome of its host — on chromosome 19 in humans.
- (However, AAV vectors do elicit a strong immune response so they can be used only once.)
As for the problem of getting the transgene to be expressed appropriately, that may be solved by using two AAV vectors simultaneously:
In the 1 January 1999 issue of Science, James M. Wilson and his colleagues reported the results of using this strategy in both mice and rhesus monkeys.
They injected their experimental animals with two vectors.
This piece of DNA contained (among other things):
- the DNA of adeno-associated virus (AAV)
- a gene encoding a protein containing two domains:
- a portion of the molecule ("p65") that is needed to activate gene transcription but that by itself cannot bind to DNA
- a portion ("FRB") that binds the drug rapamycin.
- a gene encoding another protein with two domains:
- a portion of molecule ("ZFHD1") that binds specifically to the DNA sequence in the promoter of the erythropoietin gene but that by itself cannot activate transcription of the gene;
- a portion ("FKBP12") that also binds to rapamycin.
- promoters (not shown) that allow continuous expression (transcription and translation) of the two genes. But note that, by themselves, the two gene products are inactive.
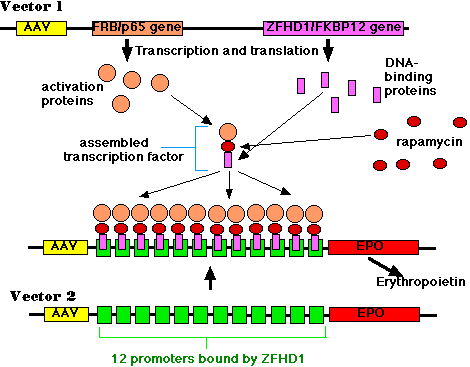
This piece of DNA contained (among other things):
- the DNA of adeno-associated virus (AAV);
- 12 identical promoters (green boxes) of the erythropoietin gene;
- the gene for erythropoietin (EPO) itself.
The experimental animals were injected (in their skeletal muscles) with many copies of both vectors. Skeletal muscle was chosen because muscle fibers are multinucleate. Once across the plasma membrane, there are many nuclei which the vectors can enter and hence many opportunities to integrate into the DNA of the host.
Later the animals were injected with rapamycin. This small molecule is an immunosuppressant and is currently being tested in transplant recipients to help them avoid rejection of the transplant. It was used here because of its ability to simultaneously bind to the FRB and FKBP12 domains of the two gene products of vector 1. The resulting trimer is an active transcription factor for the erythropoietin gene.
- injections of the two vectors had — by themselves — no effect on the production of EPO nor on the number of red blood cells (hematocrit), but
- every time these animals were given an injection of rapamycin, they
- quickly began to produce EPO (with levels increasing as much as 100 fold) and
- the number of red blood cells rose (hematocrits increasing from 42% to 60%).
- The amount of EPO produced was directly related to the amount of rapamycin given.
- Even after 5 months, a single injection of rapamycin produced a sharp rise in the level of EPO in the blood.
The results were similar to those in mice, but the effect wore off after 4 months.
So here is a system where a gene introduced into an animal can then be
- switched on by giving the animal a small molecule. (In humans, rapamycin can be given by mouth as a pill.)
- can have its output regulated by the amount of the small molecule administered.
Researchers in Seoul, Korea reported in the 23 November 2000 issue of Nature that they have used an AAV-type vector to cure
- mice with inherited IDDM (the animal equivalent of Type 1 diabetes mellitus in humans).
- rats with IDDM induced by chemical destruction of their insulin-secreting beta cells.
Both groups of animals were injected (in their hepatic portal vein) with billions of copies of a complex vector containing:
- AAV
- the complementary DNA (cDNA) encoding a synthetic version of insulin
- a promoter that is active only in liver cells and is turned on by the presence of glucose
- the DNA encoding a signal sequence (so that the insulin can be secreted)
- an enhancer to elevate expression of this artificial gene.
The results:
Both groups of animals gained control over their blood sugar level and kept this control for over 8 months. When given glucose, they proceeded to synthesize the synthetic insulin which then brought their blood glucose back down to normal levels.
Hemophilia B
The 7 December 2017 issue of The New England Journal of Medicine carried a report of a study of 10 boys injected a single time with a vector containing
- AAV
- a highly-potent human factor IX gene
- liver-specific promoter and enhancer sequences
Eight of the ten boys went a year without needing any infusions of factor IX. Since then, further trials of AAV gene therapy for hemophilia B have shown such promising results that on 22 November 2022 the US Food and Drug Administration (FDA) approved its use (to be marketed as Hemgenix).
Hemophilia A
The 28 December 2017 issue of The New England Journal of Medicine carried a report of a Phase I/II study of seven men given a single intravenous injection of an AAV vector containing the DNA encoding Factor VIII (the clotting factor that results in hemophilia A if insufficient levels are present). Link.
After three years, the six men who received the highest dose of the vector havd sufficiently high levels of Factor VIII that they have been able to stop taking injections of Factor VIII and have been mostly free of any episodes of uncontrolled bleeding.
A phase 3 clinical trial involving 132 men for 2 years has demonstrated the effectiveness and safety of this gene therapy (2023).
ALS (amyotrophic lateral sclerosis) is a human disease in which motor neurons degenerate. (It is often called "Lou Gehrig's disease" after the baseball player who died from it.)
A similar disease can be created in transgenic mice carrying mutant human genes (for superoxide dismutase) associated with ALS.
Researchers at the Salk Institute have slowed up the progression of the disease in these mice by injecting their skeletal muscles with an AAV vector containing the gene for insulin-like growth factor 1 (IGF-1).
The vector
The results: destruction of motor neurons was reduced, and the mice lived longer than they otherwise would have.
Early trials in humans indicate that the use of gene therapy for single-gene disorders is promising. In addition to the results with hemophilia A and B described above,
- On August 18, 2003, physicians in New York injected 3.5 x 109 copies of an AAV vector carrying a gene for the synthesis of GABA into the brain of a patient with Parkinson's disease. He was the first of a phase I clinical trial of this procedure. By 2007, several more Parkinson's patients had been treated with these injections with no harmful side effects and some improvement in their symptoms.
- Patients with an inherited lack of a functional gene (RPE65) needed to synthesize 11-cis-retinal — and thus destined to be blind — have had a useful level of vision restored after their eyes were given a single injection of an AAV vector containing the gene. Probably the fact that
- the vector was injected directly into the eye and so not diluted throughout the body as an intravenous injection would be;
- retinal cells rarely divide so the vector would not be lost. (The vector used had the genes needed for integration into the host cell's DNA removed so it could not be duplicated in S phase and, in dividing cells, would eventually disappear.)
- the interior of the eye is an immunologically privileged site
contributed to this remarkable success. The vector (Luxturna®) received U.S. FDA approval on 19 December 2017.
- Several children suffering from X-linked severe combined immunodeficiency have had their immune systems restored after retroviral gene therapy. [Link to discussion]
- People with the rare disorder lipoprotein lipase deficiency are unable to process the globules (chylomicrons) of fat and protein that appear in the blood after a fat-containing meal because they lack functional copies of the gene encoding lipoprotein lipase. Intramuscular injection of an AAV vector containing the functional gene provides sufficient improvement, with apparent safety, that in October 2012, this agent (Glybera®) received approval for use in the European Union. It was the first gene therapy to receive such approval.
| Link to discussions of gene therapy using
|
1 March 2023
