| Index to this page |
These cells have a number of direct functions, but they get their name from the help they provide to other types of effector cells, such as B cells and cytotoxic T lymphocytes (CTLs). The help consists of secreted cytokines that stimulate the helped cells.
| Link to drawing showing the anatomy of the lymphatic system, including the location of the thymus (52K). |
| Discussion of how antigens are presented to T cells |
 Th1 cells are produced when dendritic cells and pre-Th cells form an immunological synapse in which the dendritic cell
Th1 cells are produced when dendritic cells and pre-Th cells form an immunological synapse in which the dendritic cell
| More on dendritic cells. |
The paracrine stimulation by these cytokines causes the Th1 cells to secrete their own lymphokines:
Th2 cells are produced when antigen-presenting cells (APCs) present antigen (e.g., on parasitic helminth worms or certain allergens) to the T cell's receptor for antigen (TCR) along with
The identity of the APCs for Th2 responses is still uncertain. Some research indicates that basophils are the APCs, but other research questions this role.
The major lymphokines secreted by Th2 cells are| Link to graphic showing how Th2 cells stimulate B cells to mature into antibody-secreting plasma cells. |
Two transcription factors have been found that play a critical role in the choice between becoming a Th1 or a Th2 cell.
GATA3 produces Th2 cells by
|
The antigenic stimulus that sends pre-Th cells down one path or the other also sets the stage for reinforcing the response.
A Th1 response inhibits the Th2 path in two ways:Chemokines are cytokines that are chemotactic for (attract) leukocytes. The members of one group, who share a pair of adjacent cysteine (C) residues near their N-terminal, are designated CC chemokines.
Chemokines bind to receptors on the responding leukocyte. The receptors are transmembrane proteins with the chemokine binding site exposed at the surface of the plasma membrane. CC chemokine receptors are designated CCR.
With their different functions, we might expect that Th1 cells and Th2 cells would respond differently to chemokines. And so they do.
One chemokine that binds to CCR3 is called eotaxin. It is secreted by epithelial cells and phagocytic cells in regions where allergic reactions are occurring.
CCR3 is found on| One striking illustration: an AIDS patient with leukemia was given a bone marrow transplant from a donor whose cells expressed a nonfunctional version of CCR5. Two years later, the patient was not only cured of his leukemia but of AIDS as well. And more recently (11 April 2019), another cancer (lymphoma) patient given a bone marrow transplant from a CCR5 mutant donor was cured of his HIV-1 infection. |
| Another: Gene therapy in which samples of a patient's CD4+ T cells were treated in vitro so that their CCR5 gene became nonfunctional [how it was done]. Expanded in culture and then returned to the donor, five (of six) patients had their CD4+ T cell counts rebound. |
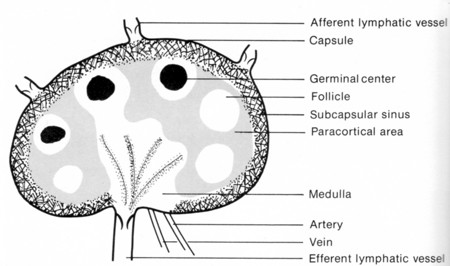
Follicular helper T cells (Tfh) are CD4+ helper T cells found in nests of B cells — called follicles — in the lymph nodes.
When exposed (in the paracortical area of the lymph node) toThe combined stimuli of antigen binding to their TCR and exposure to cytokines activate a transcription factor called Bcl-6 (first identified in a B-cell lymphoma). Bcl-6 turns on a collection of genes which, among other things, cause the Tfh cells to form an immunological synapse with those B cells expressing the antigen fragments in class II histocompatibility molecules that match their TCR.
Several other pairs of ligands and their receptors stabilize the synapse, including the interaction between CD28 on the Tfh cell and its ligand, B7, on the B cell [View].
These binding interactions stimulate the B cell to
This intense activity within the follicle forms a germinal center.
It is not yet certain whether Tfh cells represent a distinct class of Th cells or are simply a further stage in the maturation of Th1, or Th2, or Th17 cells.
Th17 cells are a recently-identified subset of CD4+ T helper cells. They are found at the interfaces between the external environment and the internal environment, e.g., skin and lining of the GI tract.
They probably start out like other "naive" Th cells, but when exposed toSituated in the skin and the lining of the GI tract, Th17 cells are positioned to attack fungi and bacteria at those locations. They do this by secreting defensins and recruiting scavenging cells, especially neutrophils, to the site. The result: clearing away of the invaders with accompanying inflammation.
But inflammation is a double-edged sword. So it is not surprising that Th17 cells have been implicated as potent effectors of such damaging inflammatory disorders as| Type | Cytokine Stimulus | Master Transcription Factor |
Effector Cytokine(s) | Main Target Cells | Effector Targets/Functions | Pathological Effects |
| Th1 | IL-12 & IL-2 | T-bet | IFN-γ & TNF-β | Macrophages, dendritic cells | Intracellular pathogens | Autoimmunity; cell-mediated allergies |
| Th2 | IL-4 | GATA3 | IL-4, IL-5 & IL-13 | Eosinophils, basophils, B cells | Various helminths | Asthma and IgE-mediated allergies |
| Tfh | IL-2 & others | Bcl-6 | IL-21 & either IL-4 or IFN-γ | B cells | Class Switch Recombination and Affinity Maturation of antibodies | IgE-mediated anaphylaxis |
| Th17 | TGF-β
plus IL-6 Inhibited by retinoic acid |
RORγt | IL-17, IL-22 & IL-23 | Neutrophils | Extracellular bacteria and fungi mediates inflammation |
Autoimmune diseases |
| pTreg | TGF-β
minus IL-6 Stimulated by retinoic acid and IL-2 |
Foxp3 | IL-10 & TGF-β | all the other types of T cells | Immunosuppression; anti-inflammatory | None? |
| Welcome&Next Search |