| Index to this page |
The strength with which an antibody molecule binds an epitope (= antigenic determinant) is called its affinity.
All the interactions between the two contribute to it. These are noncovalent interactions:Equilibrium dialysis is a technique for doing this.
The procedure requires that the epitope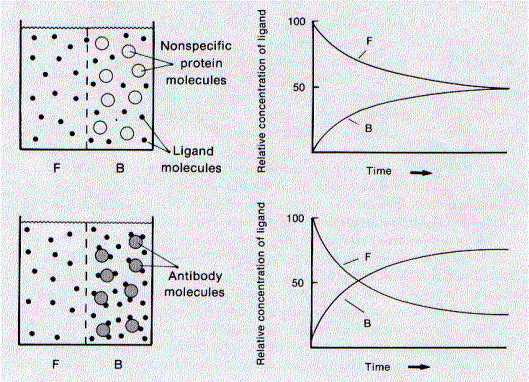 This figure shows how equilibrium dialysis performed.
This figure shows how equilibrium dialysis performed.
where the terms in brackets represent the concentration of
A more convenient form of this equation is the Scatchard equation
K = rwhere
| A | B | C | D | E | F(1) | G(2) | H(3) | I | J(4) |
| Ligand added (x 10-6M) |
"F"ront cpm |
"B"ack cpm |
(B+C) Total cpm |
(C-B) Bound cpm |
Bound ligand (x 10-6M) |
Free ligand (c) (x 10-6M) |
r | r/c (x 106) |
K (x 106 M-1) |
| 0.2 | 182 | 1044 | 1226 | 862 | 0.14 | 0.03 | 0.08 | 2.6 | 1.4 |
| 0.4 | 367 | 2021 | 2388 | 1654 | 0.28 | 0.06 | 0.15 | 2.5 | 1.4 |
| 0.8 | 739 | 3923 | 4662 | 3184 | 0.55 | 0.13 | 0.30 | 2.4 | 1.4 |
| 1.6 | 1616 | 7394 | 9010 | 5778 | 1.0 | 0.29 | 0.57 | 2.0 | 1.4 |
| 3.2 | 3952 | 14347 | 18299 | 10395 | 1.8 | 0.69 | 1.0 | 1.5 | 1.4 |
| 6.4 | 11341 | 27028 | 38369 | 15687 | 2.6 | 1.9 | 1.4 | 0.8 | 1.4 |
| "B"ack chamber contained anti-type-3 antibodies at a concentration of 1.8 x 10-6M | |||||||||
| (1) (E/D) x A | (2) (B/D) x A | (3) F/1.8 x 10-6 M | (4) r/[(2−r) x c] | ||||||
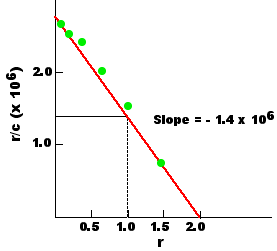
A useful way of presenting the resulting data is to plot the ratio r/c as function of r. If K is truly a constant, such a plot — called a Scatchard plot — should produce a straight line, the slope of which is − K. This Scatchard plot shows that the antibodies had an affinity for the ligand of 1.4 x 106 liters/mole (M−1).
A second useful piece of information revealed by the plot is that the maximum value for r (the x intercept) is 2. At infinitely high concentrations of ligand, each antibody molecule can bind a maximum of 2 ligand molecules; that is, the valence of these antibodies is 2. (They were IgG antibodies.)
The fact that all the antibody molecules in this experiment have the same affinity is unusual.
The immune response to most antigens produces an antiserum — a heterogeneous mixture of antibodies. Even though they all are capable of binding the antigen, they represent a pooled mixture of antibodies derived from many different clones, each with a unique B-cell receptor (BCR) — with a unique affinity — for the antigen.
In this case, the rabbits had been immunized in such a way that a single clone of antibody-secreting plasma cells had come to dominate the response. Ordinarily, only monoclonal antibodies made in the laboratory would give a Scatchard plot like this one.
This next table shows the results of equilibrium dialysis performed on a more typical antiserum. K is no longer a constant, but ranges from a value of| A | B | C | D | E | F(1) | G(2) | H(3) | I | J(4) |
| Ligand added (x 10-6M) |
"F"ront cpm |
"B"ack cpm |
(B+C) Total cpm |
(C-B) Bound cpm |
Bound ligand (x 10-6M) |
Free ligand (c) (x 10-6M) |
r | r/c (x 106) |
K (x 106 M-1) |
| 5 | 400 | 4144 | 4544 | 3744 | 4.1 | 0.44 | 0.52 | 1.2 | 0.8 |
| 10 | 1262 | 8006 | 9268 | 6744 | 7.3 | 1.4 | 0.92 | 0.68 | 0.63 |
| 15 | 2702 | 11348 | 14052 | 8644 | 9.2 | 2.9 | 1.2 | 0.40 | 0.49 |
| 25 | 6648 | 18159 | 24807 | 11511 | 12. | 6.7 | 1.5 | 0.22 | 0.41 |
| 40 | 13693 | 27380 | 41073 | 13687 | 13. | 13. | 1.7 | 0.13 | 0.40 |
| 50 | 19286 | 34091 | 53377 | 14805 | 14. | 18. | 1.8 | 0.10 | 0.40 |
| "B"ack chamber contained anti-type-3 antibodies at a concentration of 7.9 x 10-6M | |||||||||
| (1) (E/D) x A | (2) (B/D) x A | (3) F/7.9 x 10-6 M | (4) r/[(2−r) x c] | ||||||
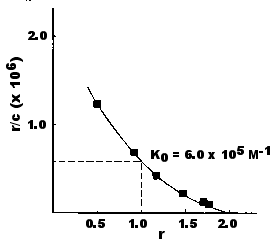
The heterogeneity of binding in this experiment is also revealed by this Scatchard plot of these data. Now the slope of the line is continuously changing as the concentration of ligand in the chambers increases.
Scatchard plots like this are the norm, not the exception, for antisera.
Despite the heterogeneity of affinities in most antisera, it is convenient to characterize such populations by defining an average affinity (K0). K0 is the value of K when the ligand concentration is such that one-half the antigen-binding sites are filled. For bivalent antibodies (like the IgG antibodies tested here), this would occur when r = 1. Thus,Thus, the average affinity is the reciprocal of the concentration of free ligand (c) when r = 1. This value can be read directly from a Scatchard plot because when r = 1, r/c = 1/c = K0. For the antiserum tested above, K0 = 6.0 x 105 M−1.

The data presented in the table and in the Scatchard plot above can be transformed according to the Sips equation
log [r/(2−r)] = a log K + a log c
where r, K and c retain their earlier definitions.
Plotting log [r/(2−r)] as a function of log c yields a straight line. (The coefficient a is slope of the line). Once again, the value for K0 can be read directly from the plot: when r =1, log [r/(2−r)] = 0. Therefore K0 is the reciprocal of that value of c where the log [r/(2−r)] = 0.
The average association constant (K0) of an antiserum tends to rise with time following immunization. This phenomenon is called affinity maturation. Presumably, as new B cells expressing new BCRs arise, they are selected for if their BCR has a greater affinity for the antigen. As they develop into a clone of plasma cells, their secreted BCRs — their antibodies — raise the K0 of the antiserum.
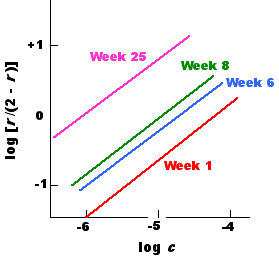
This graph shows the Sips plots of the serum samples taken at four different times from a single rabbit immunized with the Type III pneumococcal polysaccharide. The average association constant, K0, rose from 1.6 x 104 M−1 at week 1 to 6 x 105 M−1 at week 25.
Affinity maturation reflects an adaptive response to antigen exposure. As time goes by, the antibodies produced are able to bind antigen more tightly and to deal more efficiently with the antigen.
However, this is not the same as saying — as is so often seen — that the antiserum has become more specific. Often an antibody with a very high affinity for one epitope will have a substantial affinity for a related epitope as well. Such an antibody — while highly efficient — would actually have a low specificity.
The specificity of an antibody is its ability to discriminate between two different epitopes.
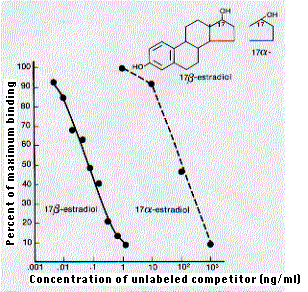
The specificity of antibodies can be so precise that they are able to discriminate between enantiomers of the same molecule. The major estrogen found in women of reproductive age is 17β-estradiol.
But as this graph [taken, with permission, from W. M. Hunter, "Radioimmunoassay" in Handbook of Experimental Immunology, 3rd ed., Vol. 1, Blackwell, 1978.] shows, the shift of the hydroxyl group on carbon 17 from the beta position (extending above the plane of the molecule) to the alpha position (extending below) lowers by 1000-fold the affinity of the molecule for antibodies raised against 17β-estradiol. (The binding was measured by radioimmunoassay.)
| Welcome&Next Search |