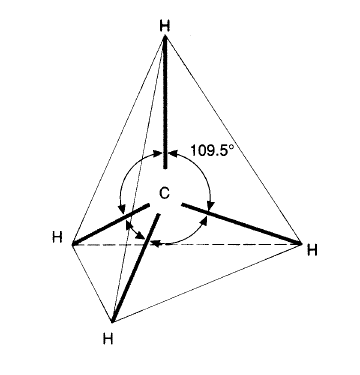
In three-dimensional (3D) space, the four covalent bonds of carbon atoms point toward the corners of a regular tetrahedron. The molecule represented to the right is methane (CH4).
Whenever a carbon atom has four different structures bonded to it, two different molecules can be formed.
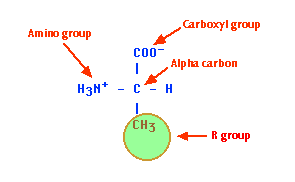
EXAMPLE: the amino acid alanine. Bonded to its alpha carbon atom are:
If you orient the molecule so that you look along it from the COO− group to the NH3+ group, the methyl (R) group can extend out
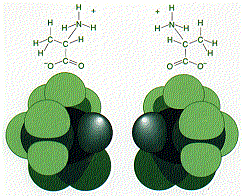
Although they share the same chemical formula, they are not interchangeable any more than a left-hand glove is interchangeable with right-hand glove.
19 of the 20 amino acids used to synthesize proteins can exist as L- or D- enantiomorphs. The exception is glycine, which has two (indistinguishable) hydrogen atoms attached to its alpha carbon.
L amino acids are used exclusively for protein synthesis by all life on our planet. (Some D amino acids are used for other purposes — view)
Does it really matter? Yes.Many other kinds of organic molecules exist as enantiomers. Usually only one form is active in biological systems. For example, if one form binds to a receptor protein on the surface of a cell, the other probably cannot.
With their protein catalysts (enzymes), cells usually synthesize only one form. However, chemical synthesis in the laboratory or pharmaceutical factory usually produces equal amounts of the two enantiomers — called a racemic mixture.
Example: The drug albuterol (e.g., Proventil®) contains equal amounts of two enantiomers. Only one of them is effective, and the other may be responsible for the occasional unpleasant side-effects associated with the drug (which is used to dilate the bronchi, e.g, during an attack of asthma). The active form can now be synthesized pure, and — called levalbuterol (Xopenex®) — is available by prescription.
Enantiomers are also called optical isomers because their solutions rotate the plane of polarized light passing through them. If one enantiomer rotates light in the clockwise direction, a solution of the other enantiomer will rotate it in the opposite direction.
| Welcome&Next Search |