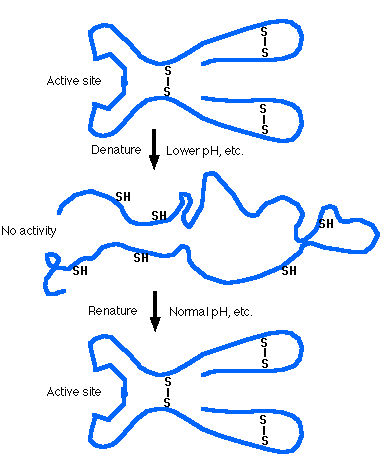
None of these agents breaks peptide bonds, so the primary structure of a protein remains intact when it is denatured.
When a protein is denatured, it loses its function.
Examples:Often when a protein has been gently denatured and then is returned to normal physiological conditions of temperature, pH, salt concentration, etc., it spontaneously regains its function (e.g. enzymatic activity or ability to bind its antigen).
This tells usTo avoid this problem, the cells of all organisms contain molecular chaperones that stabilize newly-formed polypeptides while they fold into their proper structure. The chaperones use the energy of ATP to do this work.
Some proteins are so complex that a subset of molecular chaperones — called chaperonins — is needed.
Chaperonins are hollow cylinders into which the newly-synthesized protein fits while it folds.
The inner wall of the cylinder is lined with hydrophobic amino acids which stabilize the hydrophobic regions of the polypeptide chain while it folds safely away from theChaperonins also use ATP as the energy source to drive the folding process.
As mentioned above, high temperatures can denature proteins, and when a cell is exposed to high temperatures, several types of molecular chaperones swing into action. For this reason, these chaperones are also called heat-shock proteins (HSPs).
Not only do molecular chaperones assist in the folding of newly-synthesized proteins, but some of them can also unfold aggregated proteins and then refold the protein properly. Protein aggregation is the cause of disorders such as Alzheimer's disease, Huntington's disease, and prion diseases (e.g., "mad-cow" disease). Perhaps some day ways will be found to treat these diseases by increasing the efficiency of disaggregating chaperones.
Despite the importance of chaperones, the rule still holds: the final shape of a protein is determined by only one thing: the precise sequence of amino acids in the protein.
And the sequence of amino acids in every protein is dictated by the sequence of nucleotides in the gene encoding that protein. So the function of each of the thousands of proteins in an organism is specified by one or more genes.
| There are some cases where a protein can exist in more than one conformation; that is, a given primary structure can give rise to two or more different tertiary structures. Link to an example. |
| Welcome&Next Search |