Hydrogen Bonds
Polar molecules, such as water molecules, have a weak, partial negative charge at one region of the molecule (the oxygen atom in water) and a partial positive charge elsewhere (the hydrogen atoms in water).
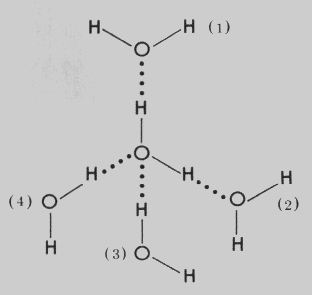
Thus when water molecules are close together, their positive and negative regions are attracted to the oppositely-charged regions of nearby molecules. The force of attraction, shown here as a dotted line, is called a hydrogen bond. Each water molecule is hydrogen bonded to four others.
The hydrogen bonds that form between water molecules account for some of the essential — and unique — properties of water.
- The attraction created by hydrogen bonds keeps water liquid over a wider range of temperature than is found for any other molecule its size.
- The energy required to break multiple hydrogen bonds causes water to have a high heat of vaporization; that is, a large amount of energy is needed to convert liquid water, where the molecules are attracted through their hydrogen bonds, to water vapor, where they are not.
Two outcomes of this:
- The evaporation of sweat, used by many mammals to cool themselves, cools by the large amount of heat needed to break the hydrogen bonds between water molecules.
- Reduction of temperature extremes near large bodies of water like the ocean.
The hydrogen bond has only 5% or so of the strength of a covalent bond. However, when many hydrogen bonds can form between two molecules (or parts of the same molecule), the resulting union can be sufficiently strong as to be quite stable.
Multiple hydrogen bonds
12 February 2011
