Secondary Structure

Most proteins contain one or more stretches of amino acids that take on a characteristic structure in 3-D space. The most common of these are the alpha helix and the beta conformation.
Alpha Helix
- The R groups of the amino acids all extend to the outside.
- The helix makes a complete turn every 3.6 amino acids.
- The helix is right-handed; it twists in a clockwise direction.
- The carbonyl group (-C=O) of each peptide bond extends parallel to the axis of the helix and points directly at the -N-H group of the peptide bond 4 amino acids below it in the helix. A hydrogen bond forms between them
[-N-H·····O=C-] . [Fig. 4.21 — right]

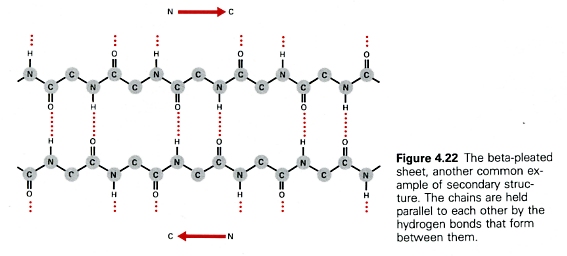
- consists of pairs of chains lying side-by-side and
- stabilized by hydrogen bonds between the carbonyl oxygen atom on one chain and the -NH group on the adjacent chain.
- The chains are often "anti-parallel"; the N-terminal to C-terminal direction of one being the reverse of the other. [Fig. 4.22 — right]
Compare:
11 April 2011



