| Index to this page |
Tertiary structure refers to the three-dimensional structure of the entire polypeptide chain.
Compare:| Primary structure | Secondary structure | Quaternary structure |

The images (courtesy of Dr. D. R. Davies) represent the tertiary structure of the antigen-binding portion of an antibody molecule. Each circle represents an alpha carbon in one of the two polypeptide chains that make up this protein. (The filled circles at the top are amino acids that bind to the antigen.) Most of the secondary structure of this protein consists of beta conformation, which is particularly easy to see on the right side of the image.
Do try to fuse these two images into a stereoscopic (3D) view. I find that it works best when my eyes are about 18" from the screen and I try to relax so that my eyes are directed at a point behind the screen.
Where the entire protein or parts of a protein are exposed to water (e.g., in blood or the cytosol), hydrophilic R groups — including R groups with sugars attached [Link] — are found at the surface; hydrophobic R groups are buried in the interior.
The normal protein has lots of alpha helical regions and is soluble. In the mutant version, the alpha helix is converted into beta conformation and the protein becomes insoluble.
Curiously, tiny amounts of the mutant version can trigger the alpha-to-beta conversion in the normal protein. Thus the mutant version can be infectious. There have been several cases in Europe of people ill with Creutzfeldt-Jakob disease that may have acquired it from ingesting tiny amounts of the mutant protein in their beef.
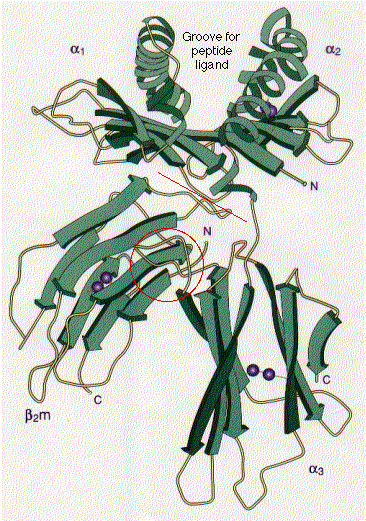
The tertiary structure of many proteins is built from several domains.
Often each domain has a separate function to perform for the protein, such as:In some (but not all) cases, each domain in a protein is encoded by a separate exon in the gene encoding that protein.
In the histocompatibility molecule shown here ,This image (courtesy of P. J. Bjorkman from Nature 329:506, 1987) is a schematic representation of the extracellular portion of HLA-A2, a human class I histocompatibility molecule. It also illustrates two common examples of secondary structure: the stretches of beta conformation are represented by the broad green arrows (pointing N -> C terminal); regions of alpha helix are shown as helical ribbons. The pairs of purple spheres represent the disulfide bridges.
A correspondence between exons and domains is more likely to be seen in recently-evolved proteins. Presumably, "exon shuffling" during evolution has enabled organisms to manufacture new proteins, with new functions, by adding exons from other parts of the genome to encode new domains (rather like Lego® pieces). [Link to gene duplication]
| Welcome&Next Search |