In 1975, NASA launched two unmanned landers towards the planet Mars:
- Viking 1 settled gently on Mars on July 20, 1976 and
- Viking 2 landed on the opposite side of the planet on September 3, 1976.
Both Viking missions carried equipment designed to look for evidence of life.
There were five different types of instruments:
No images suggesting the presence of life were ever seen.
This apparatus examined the martian soil for the presence of organic molecules. Even though sensitive to concentrations in the parts per billion (ppb) range, no organic matter was detected (except for traces of the solvents that had been used on earth to clean the equipment). Even if organic molecules could be formed on Mars, the intensity of the ultraviolet light at the surface would soon destroy them.
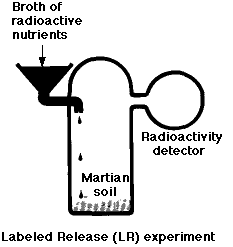 Metabolism is a universal property of life on earth. The LR experiment was designed to look for evidence of catabolism by any microorganisms that might have been present in the Martian soil.
In this experiment, a soil sample was incubated with a dilute soup of organic molecules (such as the amino acid glycine) which had been synthesized with the radioactive isotope 14C. Over a period of 10 days, the atmosphere above the sample was monitored for the appearance of radioactive gases such as carbon dioxide (CO2).
The results:
Metabolism is a universal property of life on earth. The LR experiment was designed to look for evidence of catabolism by any microorganisms that might have been present in the Martian soil.
In this experiment, a soil sample was incubated with a dilute soup of organic molecules (such as the amino acid glycine) which had been synthesized with the radioactive isotope 14C. Over a period of 10 days, the atmosphere above the sample was monitored for the appearance of radioactive gases such as carbon dioxide (CO2).
The results:
- a burst of gas production when the medium was first added
- but not when the soil had been preheated to kill off any microorganisms it might have contained.
- However, gas production did not increase as time went on (as would be expected if living organisms were growing in the medium) and
- later additions produced no additional gas.
Thus most scientists concluded that the gas was produced by nonliving chemistry (brought about by oxidizing agents in the soil). This conclusion was strengthened by similar results using soils from a dry desert in northern Chile. (See Navarro-Gonzalez, R., et al, Science, 7 November 2003)
 The PR experiment was designed to look for evidence of anabolism.; specifically whether there were any microorganisms in the martian soil that could synthesize complex organic molecules from carbon dioxide (CO2) and carbon monoxide (CO).
In this experiment, a mixture of radioactive CO2 and CO was introduced into a vessel containing a soil sample.
The PR experiment was designed to look for evidence of anabolism.; specifically whether there were any microorganisms in the martian soil that could synthesize complex organic molecules from carbon dioxide (CO2) and carbon monoxide (CO).
In this experiment, a mixture of radioactive CO2 and CO was introduced into a vessel containing a soil sample.
Because
- anabolism requires energy
- and the most important source of that energy here on earth is sunlight (for photosynthesis),
the incubation mixture was illuminated with a bright arc lamp.
After 5 days,
- any unreacted CO2 and CO was flushed out of the system and then
- the soil sample was heated to drive off any radioactive organic molecules that might have been synthesized.
The result: organic matter was detected in 7 of 9 runs. However, some positive results were achieved even on runs where the soil had first been heated to such a high temperature that any microorganisms present would have been killed (at least here on earth).
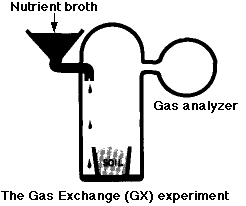 In this experiment, a known mixture of gases was placed in the chamber along with the soil sample and then analyzed periodically to see if any gases (e.g. CO2) had disappeared from — or been added to — the mixture.
In this experiment, a known mixture of gases was placed in the chamber along with the soil sample and then analyzed periodically to see if any gases (e.g. CO2) had disappeared from — or been added to — the mixture.
- In the first part of the experiment, nutrient broth was added to the chamber but not to the soil. There was a rapid release of
- large amounts of O2 (which would not be expected from heterotrophic breakdown of organic substrates). This soon subsided.
- smaller amounts of CO2 (an expected product of catabolism).
- One week later, more nutrient broth was added; this time directly to the soil. There was another, smaller, release of CO2 but no release of O2.
The conclusion: the gases were formed by nonbiological chemistry (oxidizing agents again).
The LR, PR, and GEX experiments all produced some positive results.
However:
- All of these involved puzzling ambiguities, failing to behave as similar tests done on earthly soil samples would have.
- All were later shown to be reproducible here on earth by nonbiological chemistry.
So the Viking studies probably did not reveal the presence of life on Mars.
But this is not the same as saying that life does not now nor ever did exist on Mars!
Perhaps:
- The upper layers of soil are inhospitable to life.
- Other places on Mars need to be sampled.
On 6 August 2012, NASA's Curiosity rover landed on Mars.
So far, its sampling has revealed evidence of:
The Evidence from Martian Meteorites
Some meteorites are thought — because of their peculiar chemistry — to have reached earth from Mars.
One of these ALH84001 (found in the Allan Hills of Antarctica in 1984) has been subjected to intensive analysis for ingredients suggestive of life processes.
In it have been found:
- polycyclic aromatic hydrocarbons (PAHs). But
- in most of the Martian meteorites that have been examined, these and other organic molecules have been trapped inside where no living thing could have deposited them, and
- PAHs and other organic molecules are also found in meteorites arriving from elsewhere in the solar system.
- minerals within the meteorite (e.g. carbonates, magnetite) that are formed by living organisms here on earth and appear to have been deposited in the rock of the meteorite at some later time in its history;
- objects that under the scanning electron microscope look like fossils of
tiny microorganisms. However, even the largest of these "nanofossils" have diameters of only 100 nanometers (nm) (0.1 µm, about the size of a ribosome). This is smaller than the smallest microorganisms here on earth (the mycoplasmas, with diameters of about 300 nm) and is smaller than the estimates of the minimum diameter (200 nm) needed to provide the volume necessary to build a living cell.
ALH84001 is thought to have landed in Antarctica some 13,000 years ago. But in July 2011, another Martian meteorite landed in the Moroccan desert. With much less time for terrestrial contamination to occur, it may help settle some of the controversy over the significance of the features found in ALH84001.
Organic matter is, despite its name, not the exclusive product of life. Many other meteorites contain organic matter and organic molecules can, of course, be synthesized in the laboratory from inorganic precursors.
What does distinguish the organic molecules produced by life is the restriction to one enantiomer or the other. For example, all proteins synthesized by living things here on earth use L-amino acids exclusively. Synthesis of amino acids in the chemistry laboratory produces a 50:50 mixture (called a racemic mixture) of the L- and the D- forms.
There is nothing to suggest that life couldn't work just as well with D-amino acids. What is unlikely is the ability of proteins (e.g. enzymes) to be able to function if they are made from a mixture of L- and D- enantiomers.
So if martian soil should reveal the presence of all-L (or all-D) enantiomers, this would be powerful evidence that life had produced them.
However,
- Whether tested on Mars itself, or on samples returned to earth, rigorous care must be taken to ensure that there is no contamination by terrestrial molecules.
- There is some evidence that even nonbiological synthesis in space may favor one enantiomer over the other. Four amino acids in the Murchison meteorite, which no one suggests have a biological origin, show a 7–9% excess of the L-form over the D-form.
- Over time, even in the cold, dry climate of Mars, a population of L- (or D-) enantiomers will spontaneously break down into a racemic (50:50) mixture and thus obscure a biological origin.
The case for life on Mars — whether today or in the past — is neither proven nor disproven. Stay tuned!
10 April 2015

 The PR experiment was designed to look for evidence of anabolism.; specifically whether there were any microorganisms in the martian soil that could synthesize complex organic molecules from carbon dioxide (CO2) and carbon monoxide (CO).
In this experiment, a mixture of radioactive CO2 and CO was introduced into a vessel containing a soil sample.
The PR experiment was designed to look for evidence of anabolism.; specifically whether there were any microorganisms in the martian soil that could synthesize complex organic molecules from carbon dioxide (CO2) and carbon monoxide (CO).
In this experiment, a mixture of radioactive CO2 and CO was introduced into a vessel containing a soil sample. In this experiment, a known mixture of gases was placed in the chamber along with the soil sample and then analyzed periodically to see if any gases (e.g. CO2) had disappeared from — or been added to — the mixture.
In this experiment, a known mixture of gases was placed in the chamber along with the soil sample and then analyzed periodically to see if any gases (e.g. CO2) had disappeared from — or been added to — the mixture.