

The fatty acids in fats are aliphatic hydrocarbons. If a chain holds all the hydrogen atoms it can, the molecule is said to be saturated. The fatty acids in tristearin are all saturated.
If two adjacent carbon atoms each lose a hydrogen atom, a double bond forms between them. Such a molecule is said to be unsaturated.
Ethylene is an example.
The fatty acids in trilinolein and linolenic acid are examples of unsaturated fatty acids.


The arrangement of atoms is shown on the left. The version in the center is often used to simplify diagrams of molecular structures.
The three double bonds are not restricted to the positions shown but are free to pass around the ring. This is sometimes indicated by drawing the benzene ring as it is on the far right.
Some examples of biological molecules that incorporate the benzene ring:The carotenoid, beta-carotene, is a hydrocarbon that has both aliphatic and aromatic portions.
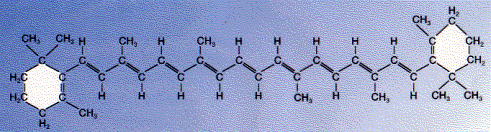
| Welcome&Next Search |