Isolating Transcription Factors
Transcription factors are extraordinarily diverse, and any one factor represents only a tiny fraction of the protein molecules present in the cell. This page describes how one can isolate and purify such rare molecules.
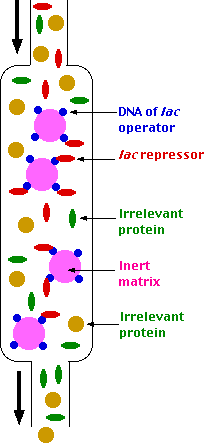
Example: isolating the lac repressor
An E. coli cell contains only 10-20 copies of the lac repressor. This represents a ratio of only 1 molecule in 50,000 protein molecules in the cell.
However, the specificity of the lac repressor for the DNA sequence of the operator provides a mechanism for fishing it out of the mixture.
The procedure:
- Learn the sequence to which the repressor binds (by footprinting).
- Synthesize a segment of DNA containing the sequence.
- Attach this artificial molecule to beads of an inert, solid medium (the matrix).
- Pour an extract of E. coli cells over the beads.
- Only molecules specific for the DNA sequence — in this case, molecules of the lac repressor — will bind to the beads.
- After irrelevant protein molecules have passed through the column,
- wash the beads with a buffer that will release the lac repressor molecules so they can be studied.
This technique is called affinity chromatography. Many eukaryotic transcription factors have also been isolated and studied by this method.
20 April 2014
