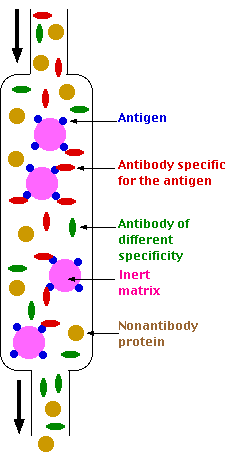
The goal of affinity chromatography is to separate all the molecules of a particular specificity from the whole gamut of molecules in a mixture such as a blood serum. For example, the antibodies in a serum sample specific for a particular antigenic determinant can be isolated by the use of affinity chromatography.
Elution. A reagent is passed into the column to release the antibodies from the immunoadsorbent. Buffers containing a high concentration of salts and/or low pH are often used to disrupt the noncovalent interactions between antibodies and antigen. A denaturing agent, such as 8 M urea, will also break the interaction by altering the configuration of the antigen-binding site of the antibody molecule.
Another, gentler, approach is to elute with a soluble form of the antigen. These compete with the immunoadsorbent for the antigen-binding sites of the antibodies and release the antibodies to the fluid phase.
Dialysis. The eluate is then dialyzed against, for example, buffered saline in order to remove the reagent used for elution.
| Link to another example of affinity chromatography. |
| Link to a discussion of another chromatographic technique: exclusion chromatography. |
| Welcome&Next Search |