Imprinted Genes
Imprinted genes are genes whose expression is determined by the parent that contributed them.
Imprinted genes violate the usual rule of inheritance that both alleles in a heterozygote are equally expressed.
Examples of the usual rule:
- If a child inherits the gene for blood group A from either parent and the gene for group B from the other parent, the child's blood group will be AB.
- If a child inherits the gene encoding hemoglobin A from either parent and the gene encoding hemoglobin S from the other parent, the child's red blood cells will contain roughly equal amounts of the two types of hemoglobin.
But there are a few exceptions to this rule. A small number of genes in mammals (~100 of them at a recent count) and in angiosperms have been found to be imprinted. Because most imprinted genes are repressed, either
- the maternal (inherited from the mother) allele is expressed exclusively because the paternal (inherited from the father) allele is imprinted or
- vice versa.
The process begins during gamete formation when
- in males certain genes are imprinted in developing sperm and
- in females, others are imprinted in the developing egg.
All the cells in a resulting child will have the same set of imprinted genes from both its father and its mother EXCEPT for those cells ("germplasm") that are destined to go on to make gametes. All imprints — both maternal and paternal — are erased in them.
Three Examples
1. IGF2
— the gene encoding the insulin-like growth factor-2
In humans (and other mammals like mice and pigs) the IGF2 allele inherited from the father (paternal) is expressed; the allele inherited from the mother is not.
If both alleles should begin to be expressed in a cell, that cell may develop into a cancer.
2. IGF2r
— the gene encoding the cell receptor for Igf-2
In mice the IGF2r allele inherited from the mother is expressed; that from the father is not. Differential imprinting accounts for this, and the mechanism is described below.
— the gene encoding the RNA that converts one of the X chromosomes in a female cell into an inactive Barr body. This process is random in the cells of the female fetus and thus is NOT an example of imprinting. However, all the cells of her extraembryonic membranes (which form the amnion, placenta, and umbilical cord) have the father's X chromosome inactivated. Imprinting of the XIST locus accounts for this.
Mechanism of parental imprinting
The process of imprinting starts in the gametes where the allele destined to be inactive in the new embryo (either the father's or the mother's as the case may be) is "marked". The mark appears to be methylation of the DNA in the promoter(s) of the gene.
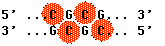
Methyl groups are added to cytosines (Cs) in the DNA. When this occurs at stretches of alternating Cs and Gs called CpG sites in a promoter, it prevents binding of transcription factors to the promoter thus shutting down expression of the gene.
| Methylation — and thus inactivation — of the promoters of tumor suppressor genes is frequently found in cancer cells. |
Although methylation seems to be the imprinting signal, keeping the gene shut down may require the production of RNA.
Example 1: the IGF2r gene
A report in Nature (16 October 1997) by Wutz et al, reveals that:
In the mother's (maternal) copy of the gene,
- there is an upstream (left) promoter that is unmethylated and active
- binding of transcription factors to this upstream promoter enables transcription of the sense strand of the gene to produce Igf2r messenger RNA.
- There is also a downstream set of CpG sites that are methylated

In the father's (paternal) copy of the IGF2r gene (the imprinted version)
- the promoter for IGF2r transcription is methylated (and inactive),
- but the downstream promoter is unmethylated and active.
- Transcription of the antisense strand from the downstream promoter produces an antisense RNA (a long noncoding RNA) that participates in shutting his gene down.
Example 2: XIST
The XIST locus on the X chromosome encodes a long noncoding RNA that shuts down all (or almost all) of the other genes on the chromosome, converting it into an inactive Barr body. [More].
Is imprinting important?
Yes.
-
Deliberate (in mice) or accidental (in humans) inheritance of two copies of a particular chromosome from one parent and none from the other parent is usually fatal (even though a complete genome is present).
- Deletion of a small portion of chromosome 15 (15q11-q13), which contains imprinted genes, causes
- a human developmental disorder called Prader-Willi syndrome when on the father's chromosome;
- a different disorder called Angelman syndrome when on the mother's chromosome.
- Failure of imprinting in somatic cells may lead to cancer.
- The cancerous cells in some cases of a malignancy called Wilms´ tumor and many cases of colon cancer have both copies of the IGF2 gene expressed (where only one, the father's, should be).
- Reduced methylation — and hence increased expression — of proto-oncogenes can lead to cancer, while
- increased methylation — and hence decreased expression — of tumor suppressor genes can also do so.
Imprinting is the reason that parthenogenesis ("virgin birth") does not occur in mammals. Two complete female genomes cannot produce viable young because of the imprinted genes.
For example, the embryo needs the father's Igf2 gene because the mother's copy has been imprinted and is inactive.
- An insulator — with a bound protein designated CTCF ("CCCTC binding factor") (named for a nucleotide sequence found in all insulators) — prevents her Igf2 gene from interacting with the enhancers needed to turn it ON.
- The father's copy of the gene can be turned on because methylation of his insulator prevents binding by CTCF so the enhancers can interact with the gene. [Link to discussion]
However, two healthy laboratory mice have been produced by parthenogenesis; that is, containing two female (haploid) genomes. (See Kono, T. et al., Nature, 22 April 2004.)
This was done by fusing two oocytes (thus each cell haploid):
- a normal oocyte with its imprinted (inactivated) Igf2 gene
- an immature oocyte
- harvested before imprinting occurs and
- containing a deletion of the insulator that blocks enhancer activation of the Igf2 gene. Thus the Igf2 gene from this oocyte could be expressed in the developing embryo.
Out of several hundred attempts, two resulting blastocysts not only implanted successfully in a surrogate mother but went on to be born normally. One even grew up and had babies of her own.
Imprinting in Plants
Some genes in the endosperm of angiosperms are imprinted by the addition of methyl groups. For some, both maternal copies (endosperm is 3n) are expressed (demethylated) while the male allele remains shut down. For other genes, it is the female alleles that are imprinted and thus not expressed while the male allele is functional.
21 January 2017
