Regeneration is the ability to replace lost or damaged body parts.
This ability varies greatly among living things.

Plants can regenerate all body parts from precursor cells. Many trees, for example, can be cut off at the ground and, in due course, sprouts appear at the margins of the stump. These go on to develop new stems, leaves, and flowers.
In the laboratory, entire plants can develop from a mass of undifferentiated cells growing in culture.
For example, a fully-differentiated carrot root cell when grown in a suitable culture medium, begins to divide repeatedly, losing its differentiated structure as it does so. Then its descendants begin to differentiate, and they finally form all the organs of a mature carrot plant.
The photo (courtesy of Roy De Carava and Scientific American) shows a carrot plant that grew in a flask from fully-differentiated root cells that had been isolated and induced to undergo mitosis.
This cnidarian can also regenerate its entire body from cells. The cells that do the job are totipotent stem cells residing in the animal's body.
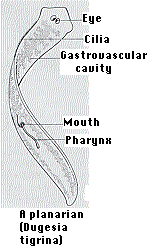

When some species of flatworms (left) are decapitated, they can regenerate a new head. Double-amputees can regenerate both a new head at the anterior surface and a new tail at the posterior surface (right). They do this by the proliferation and differentiation of the pluripotent stem cells (called neoblasts) that it retains in its body throughout its life.
How do the cells know whether to develop into a head or a tail? Thanks to the ease with which individual genes can be knocked out by RNA interference (RNAi), it has been shown that Wnt/β-catenin signaling dictates where the head and tail form.

These echinoderms can regenerate the entire organism from just one arm and the central disk.
I have read that at one time oyster fishermen used to dredge up sea stars from their oyster beds, chop them up in the hope of killing them, and then dump the parts back overboard. They soon discovered to their sorrow the remarkable powers of regeneration of these animals.
The photo (courtesy of Dr. Charles Walcott) shows a sea star regenerating an arm.

These amphibians can regenerate a missing tail, legs, even eyes. This remarkable ability is particularly pronounced in the larval stage. For this reason, larval salamanders are favorites for doing research on regeneration.
For example, cutting the tail off a larval salamander initiates the following sequence of events:The answer appears to be both.
Don't we wish that we had the same powers of regeneration that salamanders do: able to regenerate a severed spinal cord or grow a new heart!
But unfortunately, we cannot. We can regenerate some skin, a large amount of liver, and the very tips of fingers and toes. But that's about it.
Just why we are so limited is not known (but is the subject of intense research). Much of the excitement surrounding research on stem cells is because of the hope that they may provide a means of regrowing damaged or lost tissues or even organs.
In contrast to the situation that appears to hold for salamanders, dedifferention of specialized cells does not appear to play a role in the formation of a blastema in mice. Instead, the various tissues — epidermis, hair follicles, sweat glands, neurons (all ectoderm) and muscle, bone, tendon, blood vessels (mesoderm) — that participate in regenerating the tip of an amputated mouse digit (finger or toe) develop from a diverse population of "adult" stem cells in the stump that retain their restricted developmental potential. You can read about the evidence for this in Rinkevich, Y., et al., Nature, 476, 409-413 (25 August 2011).
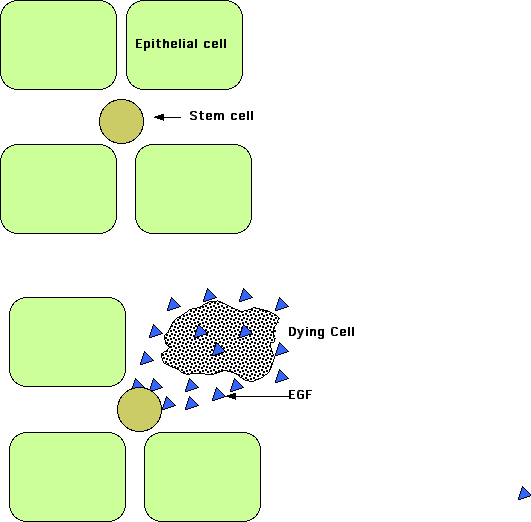
Even when an organ or tissue has not been damaged and thus does not require regeneration as such, there needs to be a way to ensure that the tissue maintains its normal complement of cells even as occasional cells die from normal attrition.
In a study done on the Drosophila intestine (Liang J. et. al., Nature, 31 August 2017), it was found that epithelial cells dying by apoptosis secrete epidermal growth factors (EGFs) that stimulate nearby stem cells in the tissue to divide and thus replace the missing cell with a fresh one. The diffusion of the EGFs extends for only about 25 μM so that replacement cells are formed only where needed.
| Welcome&Next Search |