| Index to this page |
Ozone is a highly active form of oxygen (O3 rather than O2). Ozone is made when a electric spark passes through air, and this accounts for the characteristic odor give off by some electrical motors.
Ozone presents two quite different biological problems: too much at low levels of the atmosphere (the troposphere); too little at high altitudes (the stratosphere).
Ozone is produced by the reaction of sunlight, oxygen, and automobile exhaust (which contains hydrocarbons and nitrogen oxides). Ozone is largely responsible for the discomfort associated with photochemical smog. This form of smog, long familiar to people in the Los Angeles basin, is now common wherever sunlight and stagnant air occur in urban areas (Mexico City is a dramatic example with ozone levels that often exceed 100 ppb and sometimes rise above 350 ppb). High levels of ozone during smog build-up can cause difficulty to people with respiratory ailments like emphysema and asthma. Ozone also damages plants and may be an important factor in the damage that is occurring to forests in Europe and North America.
While we often have too much ozone around us, the concentration of ozone high in the stratosphere (which begins about 7 miles [11 km] up — where airliners cruise) has declined over the past two decades. Satellite monitoring of the stratosphere, which began in 1978, has revealed a marked decline.
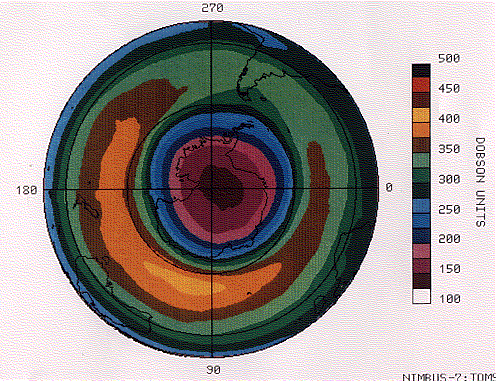
The most serious decline occurs over Antarctica in spring (October) when a precipitous drop in ozone causes an ozone hole.
The figure (courtesy of NASA) shows a map of the ozone hole measured over Antarctica on 5 October 1987 by a device carried on the Nimbus 7 satellite. The tips of South America (upper right quadrant ) and Africa (lower right) are drawn in, as is the outline of Australia and New Zealand (lower left).
The Dobson unit is a measure of the number of molecules of ozone in a vertical column of the atmosphere. You can see that the concentration of ozone decreases in ever-smaller concentric circles with the lowest reading centered over the South Pole.
Except for anomalous increases in 2015, 2020, and 2021 (attributed to volcanic eruptions in 2015 and 2021 and wildfires in Australia in 2020), the hole has been gradually shrinking. This is probably the result of phasing out the manufacture and use of chlorofluorocarbons.
The spreading of this ozone-depleted air may account for the more gradual and more protracted declines that are being seen at midlatitudes. From 1978-1990, average ozone levels declined 8% over Europe and about 5% over the United States. Smoke from recent massive wildfires (e.g. in Australia and the Pacific Northwest) can reach the stratosphere and weaken the ozone shield. These declines are ominous because ozone shields the earth's surface from much of the ultraviolet radiation reaching the earth from the sun. Ultraviolet rays can cause skin cancer, cataracts, and may depress the immune system.
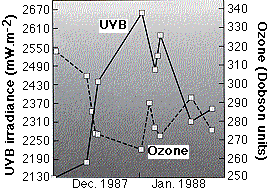 The graph (from C. R. Roy, et. al., in Nature 347:235, 1990) shows measurements of
The graph (from C. R. Roy, et. al., in Nature 347:235, 1990) shows measurements of
on several sunny days in Melbourne, Australia during December 1987 and January 1988. When ozone levels were low, ultraviolet light was more intense and vice versa. The drop in ozone, which lasted about a month, was probably caused by ozone-depleted air drifting in from the ozone hole over the South Pole. Most of the ultraviolet light that reaches the earth is ultraviolet "B" (UV-B), which includes wavelengths from 280 to 320 nm.
Their chemical inertness, which makes CFCs so desirable for industry, also makes them a threat to the atmosphere. Once in the atmosphere, it may take 60–100 years for them to decompose and disappear. In the meantime, they pose a threat to the ozone shield. Sunlight can decompose CFCs liberating chlorine atoms. These react with ozone breaking it down to O2. It is estimated that a single chlorine atom can catalyze the destruction of 10,000 ozone molecules.
Although some of the recent depletion of ozone in the stratosphere was probably due to natural causes (volcanic eruptions, fewer sunspots), some is most likely caused by chlorofluorocarbons (CFCs). However, a multi-nation agreement drawn up in 1987 — the Montreal Protocol — established a schedule for reducing, and eventually (2010) eliminating, the use of these materials. And, in fact, monitoring shows that the concentration of CFCs in the stratosphere has been decreasing since the mid-90s. However, the rate of decline slowed markedly after 2012 suggesting that some cheating (continued manufacture of CFCs) is going on.
CFCs have largely been replaced by hydrofluorocarbons (HFCs) in air conditioners and refrigerators. HFCs do not destroy ozone but are potent greenhouse gases.
While the ozone hole over the Antarctic still persists, there are signs of recovery of ozone levels at mid-latitudes (where we live). Whether this trend continues may depend on the contribution to ozone-depletion by nitrous oxide.
Nitrous oxide shares with the CFCs great stability in the stratosphere and a potent depleting effect on ozone there.
Unlike CFCs, some 60% of the N2O released to the atmosphere comes from natural sources — the decomposition of nitrogen-compounds in soil and water. However, it is estimated that human activities, primarily
The Montreal Protocol makes no mention of nitrous oxide, and it remains to be seen whether this lack needs to be corrected if ozone levels are to continue to improve.
In the troposphere, nitrous oxide is also a potent greenhouse gas (~300 times as potent as carbon dioxide).
| Welcome&Next Search |