Many humans develop allergies to simple chemicals that come in contact with their skin. A notorious example is the common allergy to urushiol, a substance found in plants of the genus Toxicodendron, including poison ivy, poison oak, and poison sumac. Urushiol is not intrinsically caustic in the way that, for example, a solution of sodium hydroxide is. Some people (including my wife) can come in contact with poison ivy without developing any skin irritation. The story is quite different for those (like me) allergic to the plant. Approximately 24 hours after contact with the plant (or even with objects, like the family dog, that have been in contact with the plant), my skin begins to redden and itch. Usually watery blisters appear. After a few days, healing begins.
The entire process is an example of an allergy: a harmful response of the body's immune system to an otherwise harmless agent. This particular form of allergy is called contact sensitivity.
Although poison ivy allergy is the most common example of contact sensitivity in North America, many other simple chemical agents can produce contact sensitivity. Metals (e.g., nickel) in jewelry are common offenders. Contact sensitivity is also a frequent problem for workers in the chemical industry.
What can be done to help those who suffer from contact sensitivity? The most obvious — and to date the most effective — is to avoid the eliciting agent. However, that is not always possible. What other ways might help?
One approach is to try a variety of different treatments (e.g., ointments) to see if any of them relieve the symptoms. This is known as the empirical approach. The history of medicine provides many examples of useful therapies that were developed empirically — that is, by trial and error.
A second approach is to design a treatment that attacks the cause of the problem. But in order to succeed in this approach, we need to know what the cause is. One of the problems associated with the "war on cancer" in the United States was that many studies were carried out before anyone had a clear understanding of what causes a cell to become cancerous and, for that matter, how the growth of a normal cell is controlled.
In order to develop a rational approach to the problem of contact sensitivity, we must learn more about it. This means experimentation. Because few humans would be willing to serve as experimental subjects for such research, biomedical scientists try to develop "animal models"; that is, to find or produce ailments in experimental animals that resemble those in humans.
An animal model of contact sensitivity was developed in the laboratories at the University of Colorado Medical School by Dr. Henry Claman, Dr. John Moorhead, and their collaborators. Their experimental animal was the mouse. Their chemical was a simple molecule called dinitrofluorobenzene (DNFB). When a drop of DNFB (dissolved in acetone and olive oil) is applied to the animal's skin, little if any visible change occurs. This is because the chemical is not intrinsically toxic or caustic. However, painting the skin with DNFB sensitizes the mouse so that it becomes allergic to the chemical. After a few days, a second application of the chemical — called the "challenge" causes the tissue changes, including swelling, that are characteristic of contact sensitivity. These investigators sensitized their animals by painting the DNFB solution on an area (about 3 cm2) of shaved skin on the abdomen.
They challenged the animals by applying a drop of the solution on each ear. In 24 hours the ears of the allergic mice become swollen in much the same way yours (or at least mine!) would if you had rubbed a leaf of poison ivy on them. The amount of swelling is measured with a micrometer (images courtesy of Dr. Moorhead).
One of the first things that these workers discovered was that intravenous injections of the same chemical (in a water-soluble form) would prevent the development of contact sensitivity. (Allergists sometimes find that allergic humans can be desensitized by injections of the same substance that causes the allergy.)
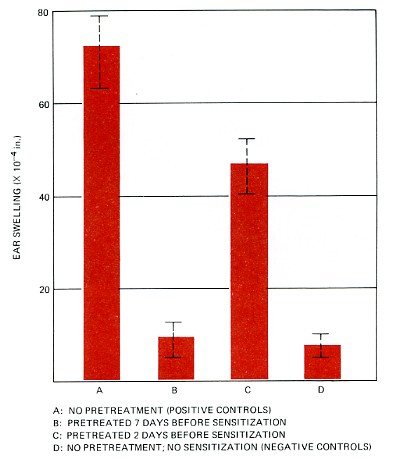
An intravenous injection of DNFB was given to one group (B) 7 days and a second group (C) 2 days before their abdomens were painted with DNFB. Then, 4 days later, all the mice had their ears challenged by painting a drop of the DNFB solution on them. This time little ear swelling occurred in group B. These mice were now "tolerant" of the chemical.
| Partial (group C) and almost complete (group B) reduction in ear swelling as a result of pretreating mice with a soluble form of DNFB. The bars represent the average (mean). The dashed lines show the 95% confidence limits — that is, the range within which there is a 95% probability that the "true" value lies. The positive controls (A) received no pretreatment and gave the maximum allergic response. The negative controls (D) were challenged on their ears but had neither been sensitized nor pretreated. (Based on the work of Phanuphak, P., Moorhead, J. W., and Claman, H. N., published in The Journal of Immunology, 112: 115, 1974.) |  |
| Link to a discussion of standard error of the mean and confidence limits. |
What could possibly account for the fact that tolerance can be induced in mice by giving them intravenous injections of a chemical that ordinarily causes them to develop contact sensitivity?
A number of explanations are possible. Perhaps the injections destroy some body constituent necessary to create the allergic state. Or perhaps the injections cause something to be produced that actively interferes with the ability of the animal to become sensitized (allergic) to the chemical.
Each of these possible explanations is a hypothesis. In the study being described, the experimenters chose to explore the second hypothesis — that is, that tolerance represents an active suppression of the machinery that normally produces allergy. How could such a hypothesis be tested?
If tolerance is an active suppression, then one might expect to find that a tolerant mouse contains something that will make other mice tolerant as well. This prediction can be tested by injecting that "something" into normal mice and then finding out if the recipients are less easily sensitized to DNFB.
But what to use?
The experimenters tried two possibilities:The results are displayed in the graph.
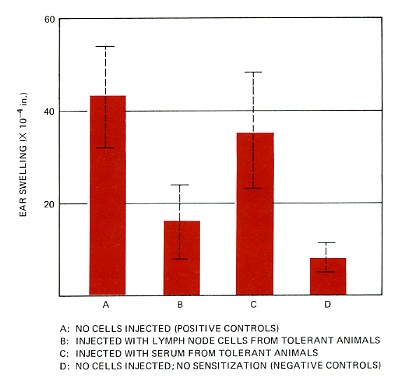 |
Normal mice can be made resistant to the development of contact sensitivity to DNFB if they are given an injection of lymph node cells from mice made tolerant to the chemical. The transfer of serum does not work. (Again, the dashed lines show the 95% confidence limits — that is, the range within which there is a 95% probability that the "true" value lies.) These results show that tolerance to DNFB is mediated by cells (that these workers called suppressor cells). Today such cells are called regulatory T cells. Data from Phanuphak, et al., The Journal of Immunology 113:1230, 1974.) |
As you can see from the graph, an injection of lymph node cells from tolerant mice appears to have conferred tolerance on the recipients. However, the injection of serum from tolerant mice appears to have had little effect. I qualify the conclusions with the words "appears to" because it is possible that chance alone produced the large effect seen in the mice in group B and, likewise, the apparent absence of an effect in group C. The likelihood of these chance events depends on such things as the sample size (6 mice were used in each group) and the fluctuations of the individual measurements about the mean.
Several statistical tests can be used to compare two sets of results such as B compared with A, and C compared with A. The question asked by all of these methods is: What is the probability that the observed differences were due to chance alone (i.e., that the experimental treatment really had no effect)? The hypothesis that the experimental treatment had no effect is known as the null hypothesis. Most workers feel that if the probability (designated p) of the observed difference is less than 1 in 20 (p = <0.05), then the null hypothesis is disproved and the observed difference is significant. In this case, the probability that the difference between group B and A was due to chance was less than 0.01. Thus the difference between group B and A was highly significant. For group C compared with group A, however, p was greater than 0.05, and thus the observed difference was not considered significant.
These results strongly support the hypothesis that tolerance in this system is caused by an active suppression — accomplished by certain cells — of the machinery that normally produces allergy. But strong support is not proof. In fact, hypotheses can never be proven to be absolutely "true" in the sense that a theorem in geometry can. The most we can say is that there is a high probability that the hypothesis provides a valid explanation of the phenomenon being studied. Hypotheses that are supported by many observations — and that can predict future observations — come to be called theories.| Welcome&Next Search |