| Index to this page |
| See Embryonic Development: Getting Started |
| see Organizing the Embryo: The Central Nervous System |
| see Organizing the Embryo: Segmentation |
The insect body plan consists of head, thorax, and abdomen. The thorax is built from three segments, T1, T2, and T3. Each carries a pair of legs; hence insects are six-legged creatures.

In most of the insect orders, T2 and T3 each carry a pair of wings (the honeybee is an example). However, flies belong to the insect order diptera; they have only a single pair of wings (on T2). The third thoracic segment, T3, carries instead a pair of balancing organs called halteres.
In Drosophila, a gene called Ultrabithorax (Ubx) acts within the cells of T3 to suppress the formation of wings. By creating a double mutation in the Ultrabithorax gene (in its introns, as it turned out), Professor E. B. Lewis of Caltech was able to produce flies in which the halteres had been replaced by a second pair of wings.

Ultrabithorax (Ubx) is an example of a "selector gene".
Selector genes are genes that regulate (turning on or off) the expression of other genes. Thus selector genes act as "master switches" in development.
Wings and all their associated structures are complicated pieces of machinery. Nonetheless, mutations in a single gene, were able to cause the reprogramming of the building of T3 (and deprived the flies of their ability to fly).
Selector genes encode transcription factors. Ultrabithorax encodes a transcription factor that is normally expressed at high levels in T3 (as well as in the first abdominal segment) of Drosophila.
These photographs were taken by, and kindly supplied by, Professor Lewis. He has spent his entire career studying selector genes in Drosophila. His life's work was honored when he shared the 1995 Nobel Prize for physiology or medicine.
When you consider the many genes that must be involved in building a complex structure like an insect leg (or wing), it is remarkable that a single gene can switch them all on. It is also clear that once a selector gene turns "on" in certain cells of the embryo, it remains "on" in all the cells derived from those cells. Those cells become irrevocably committed to carrying out the genetic program leading to the formation of a leg or wing.
Antp, Ubx, and a number of other selector genes have been cloned and sequenced. They all contain within their coding regions a sequence of some 180 nucleotides called a homeobox. The approximately 60 amino acids encoded by the homeobox are called a homeodomain. It mediates DNA binding by these proteins. Many proteins containing homeodomains have been shown to be transcription factors; probably they all are.
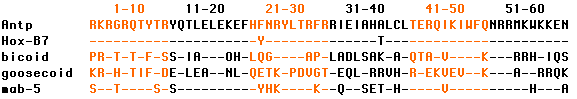
The table shows the sequence of 60 amino acids in the homeodomain of the protein encoded by the Drosophila homeobox gene Antennapedia (Antp) compared with the homeodomain encoded by the mouse gene HoxB7; by bicoid (bcd), another homeobox gene in Drosophila; by goosecoid, a homeobox gene in Xenopus; and by mab-5, a homeobox gene in the roundworm Caenorhabditis elegans. A dash indicates that the amino acid at that position is identical to the one in the Antennapedia homeobox domain. [Link to the single-letter code for the amino acids.]
Note that the mouse homeobox in HoxB7 differs from the Antp homeobox by only two amino acids (even though some 700 millions years have passed since these animals shared a common ancestor). HoxB6, used in the experiment described in the next section, differs from Antp in only 4 amino acids.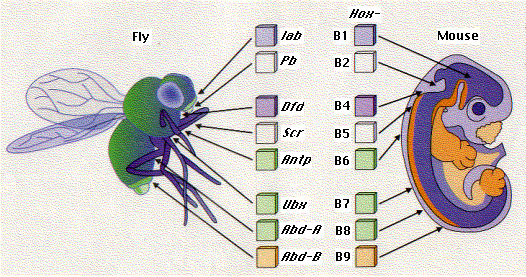
All animals that have been examined have at least one Hox cluster. Their genes show strong homology to the genes in Drosophila.
Mice and humans have 4 Hox clusters (a total of 39 genes in humans) located on four different chromosomes.All the genes in the mammalian Hox clusters show some sequence homology to each other (especially in their homeobox) but very strong sequence homology to the equivalent genes in Drosophila. HoxB7 differs from Antp at only two amino acids, HoxB6 at four.
In fact, when the mouse HoxB6 gene is inserted in Drosophila, it can substitute for Antennapedia and produce legs in place of antennae just as mutant Antp genes do.This fascinating result indicates clearly that
The foreleg of the mouse and the arm of humans contain a single upper bone, the humerus, and two lower bones, the radius and ulna. The building of the entire arm, including carpals and the phalanges of the fingers, is controlled by Hox cluster genes.
When mice were bred with homozygous mutations for both HoxA11 and HoxD11, they were born with neither radius nor ulna in the forelimbs. Here, then, is another example of the power of selector genes to initiate a whole program, perhaps involving hundreds of other genes, to form a structure as complex as a forelimb.
Mice that are homozygous for mutant HoxA10, C10, and D10 genes fail to form a lumbar and sacrum region in their vertebral column ("backbone"). Instead these vertebrae develop ribs like the thoracic vertebrae above them.
However, if any one of these 6 Hox alleles is normal, the mice are much less severely affected. This shows the high degree of redundancy of these Hox genes.
Mice have a gene, small eyes (Sey; also known as Pax6) that is similar in sequence to the Drosophila eyeless gene. As its name suggests, it, too, is involved in eye formation (even though the structure of the mouse eye is entirely different from the compound eye of Drosophila).
However, the sequences of the mouse small eyes gene and the Drosophila eyeless genes are so similar that the mouse gene can substitute for eyeless when introduced into Drosophila.
So, like the genes of the Hox clusters,
| Welcome&Next Search |