pH is a measure of the concentration of hydrogens ions (= H+) (= protons) in a solution.
Numerically it is the negative logarithm of that concentration expressed in moles per liter (M).
Pure water spontaneously dissociates into ions, forming a 10-7 M solution of H+ (and OH-). The negative of this logarithm is 7, so the pH of pure water is 7.
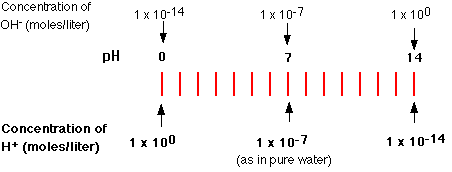
Solutions with a higher concentration of H+ than occurs in pure water have pH values below 7 and are acidic.
Solutions containing molecules or ions that reduce the concentration of H+ below that of pure water have pH values above 7 and are basic or alkaline.
Is pH important? Yes!
The properties of most proteins, enzymes for example, are sensitive to pH.
As the pH drops,The result: Not only does the net charge on the molecule change (it becomes more positive) but many of the opportunities that its R groups have for ionic interactions with other molecules and ions are altered.
As the pH rises,The result: Again the net charge on the molecule changes (it becomes more negative) and, again, many of the opportunities its R groups have for ionic interactions with other molecules or ions are altered.
| Link to an example of the importance of pH in separating serum proteins by electrophoresis. |
| Welcome&Next Search |