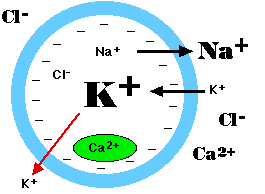
- a concentration of Na+ outside the cell that is some 10 times greater than that inside the cell
- a concentration of K+ inside the cell some 20 times greater than that outside the cell.
| Index to this page |
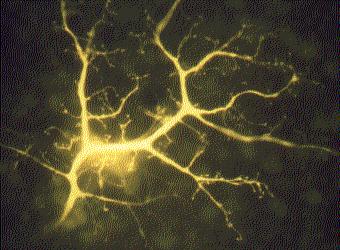
The color photo (courtesy of Julie H. Sandell and Richard H. Masland) is of a single interneuron in the retina of a rabbit. The cell has been injected with a fluorescent dye to reveal all its branches. Each of the small knobs at the tips of the branches makes a synapse with another cell in the retina.
The electric currentAll cells (not just excitable cells) have a resting potential: an electrical charge across the plasma membrane, with the interior of the cell negative with respect to the exterior. The size of the resting potential varies, but in excitable cells runs about −70 millivolts (mv).
The resting potential arises from two activities:
The sodium/potassium ATPase produces
|

|
In each case, the facilitated diffusion of sodium into the cell reduces the resting potential at that spot on the cell creating an excitatory postsynaptic potential or EPSP.
If the potential is reduced to the threshold voltage (about −50 mv in mammalian neurons), an action potential is generated in the cell.
|
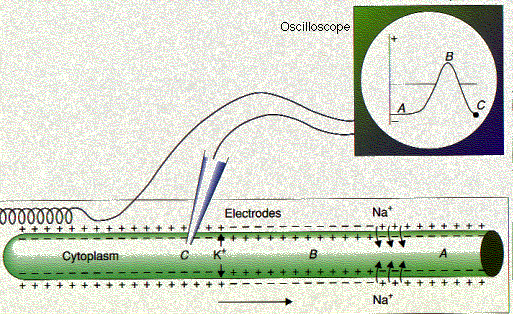
| The nerve impulse. In the resting neuron, the interior of the axon membrane is negatively charged with respect to the exterior (A). As the action potential passes (B), the polarity is reversed. Then the outflow of K+ ions quickly restores normal polarity (C). At the instant pictured in the diagram, the moving spot, which has traced these changes on the oscilloscope as the impulse swept past the intracellular electrode, is at position C. |
If depolarization at a spot on the cell reaches the threshold voltage, the reduced voltage now opens up hundreds of voltage-gated sodium channels in that portion of the plasma membrane. During the millisecond that the channels remain open, some 7000 Na+ rush into the cell. The sudden complete depolarization of the membrane opens up more of the voltage-gated sodium channels in adjacent portions of the membrane. In this way, a wave of depolarization sweeps along the cell. This is the action potential. (In neurons, the action potential is also called the nerve impulse.)
A second stimulus applied to a neuron (or muscle fiber) less than 0.001 second after the first will not trigger another impulse. The membrane is depolarized (position B), and the neuron is in its refractory period. Not until the −70 mv polarity is reestablished (position C) will the neuron be ready to fire again.
Repolarization is first established by the facilitated diffusion of potassium ions out of the cell. Only when the neuron is finally rested are the sodium ions that came in at each impulse actively transported back out of the cell.
In some human neurons, the refractory period lasts only 0.001–0.002 second. This means that the neuron can transmit 500–1000 impulses per second.
The axons of many neurons are encased in a fatty sheath called the myelin sheath. It is the greatly expanded plasma membrane of an accessory cell called the Schwann cell. Where the sheath of one Schwann cell meets the next, the axon is unprotected. The voltage-gated sodium channels of myelinated neurons are confined to these spots (called nodes of Ranvier).

The inrush of sodium ions at one node creates just enough depolarization to reach the threshold of the next. In this way, the action potential jumps from one node to the next. This results in much faster propagation of the nerve impulse than is possible in nonmyelinated neurons.
This hyperpolarization is called an inhibitory postsynaptic potential (IPSP) because it counteracts any excitatory signals that may arrive at that neuron. Although the threshold voltage of the cell is unchanged, it now requires a stronger excitatory stimulus to reach threshold.
Example: Gamma amino butyric acid (GABA). This neurotransmitter is found in the brain and inhibits nerve transmission by both mechanisms:
| More |
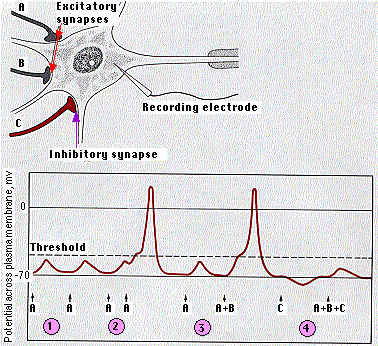
A single neuron, especially one in the central nervous system (see color photo at top), may have thousands of other neurons synapsing on it. Some of these release activating (depolarizing) neurotransmitters; others release inhibitory (hyperpolarizing) neurotransmitters.
The receiving cell is able to integrate these signals. The diagram shows how this works in a motor neuron.
Normally, the number of EPSPs needed to reach threshold is greater than shown here.
One might expect that depolarization at one point on the plasma membrane would generate an action potential irrespective of inhibitory signals elsewhere. However, this is avoided in many neurons by the axon hillock and the axon initial segment (the AIS). This is the region where the axon emerges from the cell body and is unmyelinated. The portion of the plasma membrane in this region has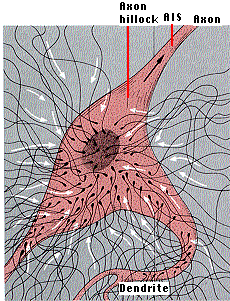
[Neurons can establish such distinctive domains on their plasma membrane by anchoring (with actin filaments) transmembrane proteins as barriers to block the free diffusion of membrane proteins from the cell body to the axon.]
The action potential is usually generated in the axon initial segment. Having neither excitatory nor inhibitory synapses of its own, it is able to evaluate the total picture of EPSPs and IPSPs created in the dendrites and cell body.
Only if, over a brief interval, the sum of depolarizing signals minus the sum of the hyperpolarizing signals exceeds the threshold of the axon initial segment will an action potential be generated.
This method for the neuron to evaluate a mix of positive and negative signals occurs rapidly. It turns out, however, that neurons also have a long-term way to integrate a mix of positive and negative signals converging on them. This long-term response involves changes in gene activity leading to changes in the number and activity of the cell's many synapses.
| Link to discussions of long-term facilitation and long-term depression. |
| Welcome&Next Search |